NIV is increasingly used for prevention and treatment of respiratory complications and failure. Some of them are admitted to the PACU with advanced hemodynamic monitors which allow quantification of extravascular lung water (EVLW) by transpulmonary thermodilution technique (TPTD) and pulmonary vascular permeability (PVP) providing information on lung edema.
AimThe objective of this study was to ascertain if EVLW index and PVP index may predict failure (intubation) or success (non-intubation) in patients developing acute respiratory failure (ARF) in the postoperative period following major abdominal surgery, where the first line of treatment was non-invasive continuous positive airway pressure via a helmet.
MethodsHemodynamic variables, EVLWI and PVPI, were monitored with a transpulmonary thermodilution hemodynamic monitor device (PiCCO™) before and after the application of CPAP.
ResultsAvoidance of intubation was observed in 66% of patients with Helmet-CPAP. In these patients after the first hour of application of CPAP and PaO2/FiO2 ratio significantly increased (303.33±65.2 vs 141.6±14.6, P<.01). Before starting, Helmet-CPAP values of EVLWI and PVPI were significantly lower in non-intubated patients (EVLWI 8.6±1.08 vs 11.8±0.99ml/kg IBW, P<.01 and PVPI 1.7±0.56 vs 3.0±0.88, P<.01). An optimal cut-off value for EVLWI was established at 9.5, and at 2.45 for PVPI (sensitivity of 0.7; specificity of 0.9, P<.01).
ConclusionIn this type of patient the physiological parameters that predict the failure of Helmet-CPAP with the greatest accuracy were the value of the EVLWI and PVPI before Helmet-CPAP institution and the PaO2/FiO2 ratio and the respiratory rate after 1h of CPAP.
La ventilación no invasiva (VNI) se utiliza cada vez más para la prevención y el tratamiento de las complicaciones y la insuficiencia respiratorias. Algunos pacientes ingresan en las unidades de reanimación postanestésica portando monitores hemodinámicos avanzados que permiten cuantificar el agua pulmonar extravascular (EVLW) mediante la técnica de termodilución transpulmonar y la permeabilidad vascular pulmonar (PVP), parámetros que permiten obtener información sobre el edema pulmonar.
ObjetivoEl objetivo de este estudio fue determinar si el índice de EVLW y el índice de la PVP pueden pronosticar el fracaso (intubación) o el éxito (no intubación) en pacientes que desarrollan insuficiencia respiratoria aguda (IRA) durante el período postoperatorio de una intervención quirúrgica mayor abdominal y cuyo tratamiento de primera línea es la presión positiva continua en la vía aérea (CPAP) administrada mediante casco (CPAP-Helmet).
MétodosSe monitorizaron las variables hemodinámicas, el índice de agua pulmonar extravascular (EVLWI) y el índice de permeabilidad vascular pulmonar (PVPI) mediante un dispositivo de monitorización hemodinámica de termodilución transpulmonar (PiCCO™), antes y después de la aplicación de la CPAP.
ResultadosEn un 66% de los pacientes con CPAP-Helmet se evitó la intubación. En dichos pacientes, el cociente PaO2/FiO2 aumentó de forma significativa (303,33±65,2 vs. 141,6±14,6, p<0,01) tras la primera hora de aplicación de la CPAP. Antes de iniciar la CPAP-Helmet los valores de EVLWI y PVPI eran significativamente inferiores en los pacientes no intubados (EVLWI 8,6±1,08 vs. 11,8±0,99ml/kg de peso corporal ideal (PCI), p<0,01 y PVPI 1,7±0,56 vs. 3,0±0,88, p<0,01). Se establecieron unos valores de corte óptimos de 9,5 para el EVLWI y de 2,45 para el PVPI (sensibilidad de 0,7; especificidad de 0,9, p<0,01).
ConclusiónEn este tipo de pacientes, los parámetros fisiológicos que pronosticaron el fracaso de la CPAP-Helmet con mayor precisión fueron el EVLWI y el PVPI previos al inicio de la CPAP-Helmet, el cociente PaO2/FiO2 y la frecuencia respiratoria tras una hora de CPAP.
Non-invasive positive pressure ventilation (NPPV) has improved the management of respiratory failure, particularly in patients with heart failure.1 Patients receiving NPPV demonstrate both clinical and blood gas improvements. In the majority of cases, NPPV also eliminates the need for intubation and invasive mechanical ventilation.2
Continuous positive airway pressure may stimulate clearance of pulmonary edema through various mechanisms. The most obvious mechanism would be an increase in functional residual capacity, thus enlarging the surface area for alveolar-capillary exchange, which would contribute to the reabsorption capacity of edema fluid in the lungs.3
In patients admitted to post-anesthesia care units after major surgery, NPPV is increasingly used for prevention and treatment of respiratory complications and failure.4–6 Randomized studies have shown that continuous positive airway pressure reduces atelectasis and prevents pneumonia more effectively than standard therapy after upper abdominal surgery,7 and that non-invasive ventilation (NIV) significantly improves gas exchange and pulmonary function abnormalities after procedures such as thoracic, cardiac, vascular surgeries and liver resection.7 These studies support the use of continuous positive airway pressure or NIV in the postoperative setting, but more studies are required before specific recommendations can be made.
High-risk surgical patients are managed intra- and postoperatively with advanced hemodynamic monitoring.8 Several studies have shown that goal-directed hemodynamic therapy (GDT) and fluid optimization may result in improved outcomes.9,10 These advanced monitoring systems guide postoperative therapy in patients admitted to the post-anesthesia care unit.
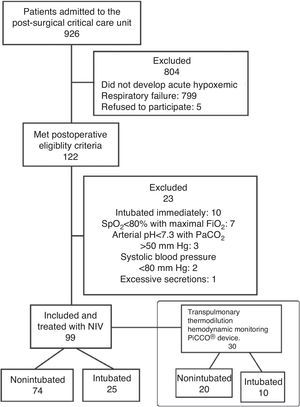
The PiCCO™ device (Pulsion, Medical Systems, Munich, Germany) quantifies extravascular lung water index (EVLWI) by transpulmonary thermodilution (TPTD), which provides information on the magnitude of the edema as well as tracking its evolution.11 Another parameter is the pulmonary vascular permeability index (PVPI) which determines the cause of pulmonary edema: high PVPI indicates increased lung permeability (as in ARDS or sepsis), while normal values are present in hydrostatic pulmonary edema (heart failure and volume overload).12,13 However EVLWI and PVPI values have not been related to the onset and evolution of postoperative respiratory failure. We hypothesized that pre-NPPV EVLWI values could be related to the failure (intubation) or success (non-intubation) of NPPV in this setting. Using data from a previous study,6 we analyzed the evolution of EVLWI and PVPI values in 30 patients with ARF following major abdominal surgery, where the first line of treatment was continuous positive airway pressure applied via a helmet (helmet continuous positive airway pressure).6
Materials and MethodsData for the present analysis were extracted from a previous retrospective study6 of 99 patients admitted to the post-anesthesia care unit after mayor surgery. All these patients developed clinical signs of multi-etiological acute respiratory distress, and were treated with helmet continuous positive airway pressure.
The original study was approved by the Clinical Research Ethics Committee of the Hospital General Universitario of Ciudad Real, Spain (Chairperson Dr. Teresa Rodriguez Cano) (Ethics Committee No. PI-207/01) on 11 September 2007. Clinical data were obtained from the standard medical records of patients treated in the post-anesthesia care unit.
Indications for continuous positive airway pressure included hypoxemia (PaO2/FiO2 ratio of less than 200mmHg while spontaneous breathing with supplemental oxygen by facemask), respiratory acidosis (PaCO2 >45mmHg and pH <7.35), respiratory rate>24breaths/min, and presence of severe dyspnea and contraction of the accessory inspiratory muscles or paradoxical abdominal motion.
From this population, only those who were monitored with the PiCCO™ device via transpulmonary thermodilution were included for the analysis (Fig. 1).
NPPV TechniqueContinuous positive airway pressure was administered via a helmet (CaStar, Starmed, Italy)14 using different levels of pressure (cmH2O) according to the degree of dyspnea and hypoxemia. Pressure was generated using a flow generator with adjustable inspiratory oxygen fraction, and delivered through a latex-free polyvinyl chloride transparent helmet at different pressure readings. A flow meter allowed high flows to be administered, with mixtures of O2 and air.
The fraction of inspired oxygen (FiO2) was adjusted to obtain an oxygen saturation of SpO2 >92%. A heat-moisture exchanger filter was placed at the outlet to reduce dryness and to diminish the noise inside the helmet.
Helmet continuous positive airway pressure success was defined as the avoidance of endotracheal intubation and invasive mechanical ventilation after 24h. NPPV failure was defined as the requirement for endotracheal intubation. The decision to perform endotracheal intubation was made by the attending physician according to the usual criteria used in the unit, i.e., the inability to maintain a PaO2/FiO2 ratio >100 for 1h of NPPV, cardiac arrest, respiratory arrest, respiratory pauses with loss of consciousness, severe encephalopathy, agitation not controlled by sedation, shock, intolerance of continuous positive airway pressure (discomfort or claustrophobia), or deterioration in gas exchange.15
Data CollectionThe following data were collected from the records: demographic data, simplified acute physiology score (SAPS) II, sepsis-related organ failure assessment (SOFA), associated co-morbidities (cardiovascular, respiratory), surgery time, hemoglobin and body temperature. The type of surgery (head and neck, esophagectomy+gastrectomy, morbid obesity, lower abdomen, thoracic surgery, aortobifemoral bypass, and trauma surgery) and the cause of the acute respiratory failure (ARDS, acute cardiogenic pulmonary, pneumonia, atelectasis, exacerbations of COPD and pleural effusion) are shown in the previous study.6
Respiratory and hemodynamic data were collected before and immediately after start of Helmet-CPAP, and at 1, 3, 6, 12, 24 and 48h and at intermediate intervals if noted in the records.
Respiratory data included arterial blood gases (PaO2, PaCO2, HCO3−, pH), PaO2/FiO2 ratio and respiratory rate.
Hemodynamic parameters included heart rate, systolic, diastolic and mean arterial pressure, cardiac index (CI), intrathoracic blood volume (ITBV), EVLWI indexed to the ideal body weight, PVPI, systemic vascular resistance index (SVRI) and the surrogate for left ventricular contractility (dPmax). All were recorded from data displayed on the PiCCO™ monitor.
Quantification of EVLWI measured via transpulmonary thermodilution (TPTD) on the PiCCO™ device indicates the magnitude of the edema and monitors its evolution. Another parameter shown by this device is the pulmonary vascular permeability index (PVPI), which is measured as the ratio of EVLWI to pulmonary blood volume. It has been used to determine the cause of pulmonary edema: high PVPI indicates increased lung permeability due to an inflammatory process (such as ARDS), while normal values are present in hydrostatic pulmonary edema (heart failure and volume overload).11,12
StatisticsNominal variables were expressed as mean±SD (standard deviation) and processed as continuous variables. Qualitative variables between two groups were compared using Fisher's exact test or the χ2 test. For quantitative variables, comparisons were performed using the Mann Whitney U test. Analysis was made with a non-parametric paired Wilcoxon test to compare data obtained before and after NPPV for each patient. A P value of <.01 was considered statistically significant. Receiver operating characteristic (ROC) curves were used to analyze the best predictor for postoperative respiratory failure. All statistical analyses were performed using SPSS 11.0.1 statistical software.
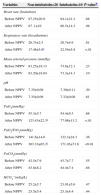
ResultsOf the 99 patients managed with helmet-NPPV for postoperative ARF included in the previous study,6 30 had been monitored with PiCCO™ and were therefore included in this study. The characteristics of these patients on admission to the post-anesthesia care unit are shown in Table 1. There were no statistically significant differences in the characteristics of patients who were subsequently intubated (intubated patients) and those that were not (non-intubated patients).
Patient Characteristics on Admission to the Unit.a
| Characteristics | Non-intubatedn=20 | Intubatedn=10 | P-value |
|---|---|---|---|
| Age, years | 60.955±18.42 | 59.25±15.2 | .61 |
| Male/female, n | 12/8 | 6/4 | .81 |
| Cardiac disease | 14 (70) | 6 (56) | .44 |
| Respiratory disease | 9 (45) | 5 (50) | .55 |
| Body temperature (°C) | 37.145±0.44 | 37.185±0.65 | .74 |
| Hemoglobin (g/dl) | 11.35±1.7 | 10.95±1.5 | .53 |
| Surgery (h) | 3.6±1.3 | 4.7±2.3 | .25 |
| SAPS II score | 41.7±15.4 | 37±15.5 | .68 |
| SOFA score | 4.4±1.3 | 4.9±0.6 | .61 |
SAP II, simplified acute physiology score; SOFA, sequential organ failure assessment.
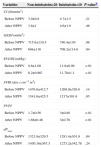
Changes in physiological parameters (heart rate, respiratory rate and mean arterial pressure) and arterial blood gases obtained before and after NPPV are shown in Table 2. After 1h of NPPV, respiratory frequency decreased significantly only in the non-intubated group (26.33±2.5 vs 17.48±5.8, P<.01). After NPPV, the intubated group had a significantly lower PaO2/FiO2 ratio (141.6±14.6 vs 303.33±65.2, P<.01).
Changes in Clinical Parameters and Arterial Blood Gases Before and After 1h of NPPV.a
| Variables | Non-intubatedn=20 | Intubatedn=10 | P-valueb |
|---|---|---|---|
| Heart rate (beats/min) | |||
| Before NPPV | 97.25±20.9 | 94.1±21.2 | .68 |
| After NPPV | 87. 1±16 | 88.5±14.3 | .98 |
| Respiratory rate (breaths/min) | |||
| Before NPPV | 26.33±2.5 | 28.7±4.9 | .81 |
| After NPPV | 17.48±5.8c | 22.54±5.8 | <.01 |
| Mean arterial pressure (mmHg) | |||
| Before NPPV | 83.25±19.13 | 73.8±12.1 | .23 |
| After NPPV | 83.20±18.04 | 73.3±14.1 | .10 |
| pH | |||
| Before NPPV | 7.35±0.09 | 7.30±0.11 | .30 |
| After NPPV | 7.35±0.09 | 7.32±0.08 | .61 |
| PaO2(mmHg) | |||
| Before NPPV | 55.3±5.7 | 54.4±9.3 | .68 |
| After NPPV | 123.43±22.3c | 77.98±13.2 | <.01 |
| PaO2/FiO2(mmHg) | |||
| Before NPPV | 141.6±14.6 | 132.3±24.3 | .36 |
| After NPPV | 303.33±65.2c | 171.03±71.6 | <0.01 |
| PaCO2(mmHg) | |||
| Before NPPV | 42.5±7.9 | 43.7±7.7 | .55 |
| After NPPV | 43.8±8.2 | 44.4±7.4 | .88 |
| HCO3−(mEq/L) | |||
| Before NPPV | 25.2±5.7 | 23.01±5.6 | .47 |
| After NPPV | 25.5±5.4 | 23.3±4.4 | .44 |
Analysis of the ROC curve showed a very good predictive capacity for respiratory frequency and PaO2/FiO2 ratio, with an area under the curve of 0.91 (95 CI, 0.80–1.01, P<.01) and 0.85 (95% CI, 0.72–0.99, P<.01) respectively (Fig. 2). A higher level of CPAP-cmH2O (10.42±1.03 vs 13.96±1.7, P<.01) and higher FiO2 (0.42±0.04 vs 0.49±0.06, P<.01) was applied in the intubated group vs the non-intubated group.
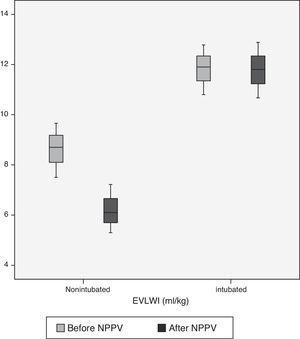
The hemodynamic parameters from the PiCCO™ monitor obtained before and 1h after NPPV are shown in Table 3. The non-intubated group had significantly lower pre-NPPV EVLWI than the intubated group (8.6±1.08 vs 11.8±0.99, P<.01). However, after 1h of NPPV, EVLWI only decreased significantly in the non-intubated group (8.6±1.08 vs 6.2±0.96, P<.01) accompanied by a significantly lower PVPI (1.7±0.56 vs 3.0±0.88, P<.01). After NPPV, this difference was maintained in both groups (1.68±0.46 vs 3±0.76, P<.01).
Changes in Hemodynamic Parameters (PiCCO®) Before and After 1h of NPPV.a
| Variables | Non-intubatedn=20 | Intubatedn=10 | P-valueb |
|---|---|---|---|
| CI (l/min/m2) | |||
| Before NPPV | 3.9±0.8 | 4.7±1.5 | .21 |
| After NPPV | 3.8±1 | 4.6±1.9 | .46 |
| GEDI (ml/m2) | |||
| Before NPPV | 715.6±110.5 | 740.4±130 | .98 |
| After NPPV | 694±116 | 709.2±114.6 | .94 |
| EVLWI (ml/kg) | |||
| Before NPPV | 8.6±1.08 | 11.8±0.99 | <.01 |
| After NPPV | 6.2±0.96c | 11.78±1.1 | <.01 |
| SVRI (dynscm−5m2) | |||
| Before NPPV | 1476.6±412.7 | 1269.9±326.6 | .14 |
| After NPPV | 1541.6±425.5 | 1217±391.6 | .05 |
| PVPI | |||
| Before NPPV | 1.7±0.56 | 3±0.88 | <.01 |
| After NPPV | 1.68±0.46 | 3±0.76 | <.01 |
| dPmax | |||
| Before NPPV | 1321.8±329.5 | 1281.4±301.9 | .84 |
| After NPPV | 1430.16±367.3 | 1273.2±342.78 | .24 |
EVLWI, extravascular lung water index; GEDI, global end-diastolic volume index; SVRI, systemic vascular resistance index; dPmax, left ventricular contractility; CI, cardiac index; PVPI, pulmonary vascular permeability index.
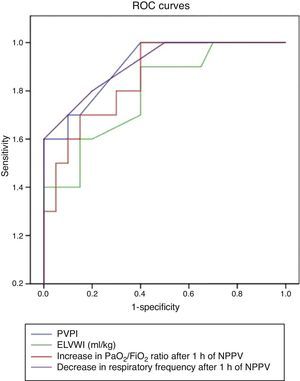
When the EVLWI and PVPI were included in ROC curve analysis (Fig. 3), both variables showed very good predictive capacity, with an area under the curve of 0.8 and 0.9, respectively. An optimal cut-off value for EVLWI was established at 9.5 (sensitivity 0.78; specificity 0.8, P<.01), and at 2.45 for PVPI (sensitivity 0.7; specificity 0.9, P<.01).
DiscussionIn our study, the application of NPPV with continuous positive airway pressure in patients with postoperative ARF was associated with an improvement in gas exchange, and avoided the need for intubation in 66.6% of the patients. We found for the first time that pre-NPPV EVLWI and PVPI values predicted the failure of NPPV with an accuracy similar to changes in PaO2/FiO2 ratio and respiratory frequency after 1h of NPPV. An EVLWI value of 9.5ml/kg and a PVPI of 2.45 predicted the need for intubation with high sensitivity and specificity.
Various studies have shown that application of continuous positive airway pressure in postoperative ARF improves gas exchange, increases functional residual capacity, and minimizes the formation of atelectasis.16,17 The use of NPPV relieves work of breathing, reduces pulmonary extravascular water,24 and enlarges pulmonary volume by re-expanding areas of atelectasis.25 The role of NPPV in the management of ARF is gaining ground in the literature.18–21
In this study, we found an improvement in oxygenation and a reduction in respiratory frequency after NPPV, which is consistent with the results obtained in the context of ARF.16,22 Indeed, our success rate and gas exchange improvements were similar to those described in patients with postoperative ARF following abdominal surgery.5,6
If we compare the intubated group with the non-intubated group, we observed that the post-NPPV PaO2/FiO2 ratio in the non-intubated group presented a statistically significant improvement. This could indicate a reversal in areas of atelectasis, which did not occur in the intubated group (303.33±65.2 vs 171.03±71.6).
The helmet has been shown to be an effective interface for the application of NPPV; however, it can increase patient ventilator asynchrony and CO2 rebreathing when compared to the facemask. The helmet, however, is better tolerated, and can thus be used for longer periods.23 In this study, PaCO2 increased non-significantly in the non-intubated group (42.5±7.9 vs 43.8±8.2) compared to the intubated group (43.7±7.7 vs 44.1±7.4) 1h after start of NPPV; however, the changes were clinically insignificant (Table 2).
Extravascular lung water is a valuable tool for measuring pulmonary edema before it is clinically evident, and for guiding therapy with electrolyte solutions and vasoconstrictors in the critically ill patient.24 In the future, it may be included in the definition of acute lung injury (ALI) and ARDS.25 Several studies have demonstrated that EVLWI is an independent predictor of mortality in septic patients with or without ARDS.26–28
PVPI has been shown to be useful in differentiating hydrostatic edema due to left ventricular failure (normal values of PVPI <3) from high permeability edema due to ARDS (higher PVPI values >3).29–32 Increased PVPI values indicate possible changes in the permeability of the alveolar-capillary membrane as a consequence of an inflammatory process and lung injury, while higher EVLWI would indicate edema. In these cases, the probability of NPPV failure is higher when compared to patients with lower (normal) PVPI and EVLWI without lung injury.
In our study, pre-NPPV EVLWI and PVPI values (1.7±0.56 vs 3±0.88, P<.01) were significantly higher in the intubated group (8.6±1.08 vs 11.8±0.99, P<.01). In the non-intubated group, EVLWI decreased (8.6±1.08 vs 6.2±0.96, P<.01) after start of NPPV, but values remained within normal range. The intubated group, however, had higher initial values that did not change after NPPV (11.8±0.99 vs 11.7±1.1, NS). The same was observed in the case of PVPI (3.0±0.88 vs 3±0.76, NS).
Gust et al.16 analyzed the effects of NPPV in patients after coronary bypass surgery. They found that EVLWI was significantly lower in patients on CPAP or BiPAP™ compared to the group that remained on O2 alone. Nevertheless, other studies27 indicate that EVLWI does not diminish or can even increase after the application of positive pressure.
Results from other series cannot be interpreted without knowledge of PVPI values. However, we hypothesize that in patients with normal permeability, EVLWI may be reduced when alveolar surface area is increased with continuous positive airway pressure. In ARDS with high permeability edema, meanwhile, positive pressure would not affect PVPI or EVLWI.
The ROC curve shows that the model has prognostic value (greater EVLWI and PVPI predict a greater incidence of intubation after PNNV). For EVLWI, the optimal cut-off point to predict need for intubation was 9.5. For PVPI, this value was 2.45. Based on these results, early intubation and immediate mechanical ventilation would be indicated in the presence of these EVLWI and PVPI values. This is because the increase in extravascular lung water is not due to increased hydrostatic pressure, but rather to altered vascular permeability with a possible inflammatory origin that will only resolve over time.
Our study has some limitations. First of all, it is a sub-analysis of a retrospective observational study with no control group. Therefore, we are unable to conclude that the results seen in these subjects were modified by the use of NPPV. However, our study explores the possible causal relationship of high EVLWI and the failure of non-invasive management of the patients. Our descriptive study could provide the basis for a wider prospective randomized clinical trial to confirm our preliminary findings. Another limitation is our small sample size, which restricts the accuracy of PVPI and EVLWI cut-off values, sensibility and specificity. Finally, the study is limited to patients with access to a PiCCO; most patients on NPPV are monitored with non-invasive or less invasive techniques that do not measure EVLWI and PVPI index.
ConclusionsIn patients with postoperative ARF, the physiological parameters that predict the failure of NPPV with the greatest accuracy are PaO2/FiO2 ratio and respiratory frequency. However, in this small pilot study, we have seen a good predictive value for pre-NPPV EVLWI and PVPI. High EVLWI and PVPI values indicate an alteration in the alveolar-capillary membrane and could suggest when NPPV could be avoided in favor of early intubation. A prospective randomized clinical trial would be necessary to confirm these results.
Conflicts of InterestFJB is a member of the Medical Advisory Board of Pulsion Medical Systems, Munich, Germany.
We thank the nursing staff on the unit for their kind and generous help, without which this work would not have been possible.
Please cite this article as: Redondo Calvo FJ, Bejarano Ramirez N, Uña Orejon R, Villazala Garcia R, Yuste Peña AS, Belda FJ. La elevación del índice de agua pulmonar extravascular como factor predictivo del fracaso de la presión continua en la vía aérea con casco (CPAP-Helmet) en pacientes con insuficiencia respiratoria aguda tras intervención quirúrgica mayor. Arch Bronconeumol. 2015;51:558–563.