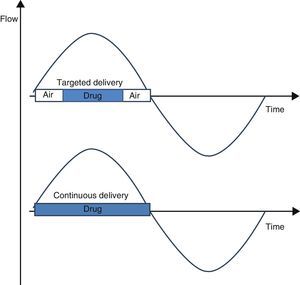
It has been difficult to develop more effective nebulizers for the improved delivery of drugs directly to the lungs. Adaptive nebulizers were designed for the administration of drugs at a specific time during inspiration, depending on the intended target (drug-targeting nebulization). Controlled inhalation nebulizers, such as AKITA®, have recently become another addition to adaptive aerosol delivery systems.1 The aim of our study was to measure the inhaled dose and pulmonary bioavailability obtained from drug-targeting nebulization, compared to conventional continuous nebulization.
We used the AKITA® delivery device (Activaero; Germany) that provides individualized, controlled flow and inhalation volumes in several puffs. Aerosolized drug delivery was either continuous or targeted (Fig. 1) during each 4-s controlled inhalation. The number of inhalations was 43 or 86, respectively.
The inhaled dose of a solution of amikacin (125mg/ml) was measured in vitro using a residual gravimetric method.2 Five healthy non-smoking male volunteers (mean age 27.8±4.7 years) were selected, and approval was obtained from the ethics committee.
Subjects were randomized to receive a solution of salbutamol (GlaxoSmithKline, Belgium) (625mg/ml) via continuous or targeted nebulization.
Subjects swallowed 100ml of activated charcoal before and after nebulization (Carbomix, Norit, Netherlands). Urine samples were obtained before nebulization and 30min after (Cu-30), and then from each spontaneous micturition and 240min (Cu-240) after the start of nebulization. The volume of each micturition was measured.
Salbutamol levels in urine were measured in triplicate by HPLC.3 The amount of salbutamol excreted in urine (Cu) was calculated by multiplying the concentration by the volume of each sample. Pulmonary bioavailability was compared using the Cu-30 sample.
Mean anthropometric and spirometric values were as follows: height 177.6±8.8cm and weight 80.0±19.6kg, FVC 103.7%±16.8% of the predicted value and FEV1 100.3%±14.5% of the predicted value.
The inhaled dose was not significantly different (15.8% versus 16.5%, respectively, p=0.975) but the amount of drug delivered with the continuous nebulization method was twice that of the targeted method (p<0.001).
The excreted amounts of salbutamol are summarized in Table 1. Cu-30 was similar for both delivery systems (p=0.947). The accumulated amount of salbutamol excreted in urine was significantly higher with the targeted nebulization system (p<0.05).
Excreted Salbutamol (mcg) Retrieved After Nebulization With the Continuous or Discontinuous Delivery System in 5 Healthy Individuals.
| Continuous | Discontinuous | |
| Cu-30 | 36.69±31.56 | 35.56±28.39 |
| Cu-60 | 30.59±15.63 | 51.72±37.24 |
| Cu-240 | 51.08±25.87 | 84.89±36.47 |
| Accumulative amount | 118.36±47.77 | 172.18±46.82* |
Results expressed as mean±standard deviation.
Pulmonary bioavailability of nebulized salbutamol is a reproducible measurement for predicting drug deposition in the lung4 and for determining the amount of drug that is provided to the body via the pulmonary route and rapidly excreted in the urine. Concentrations may be overestimated in healthy individuals,5 but this does not affect the comparison of the delivery methods. Our results confirm that the AKITA® device delivers a greater amount of drug than other devices, with a pulmonary bioavailability approximately twice that previously described with 8 conventional nebulizers with different flows.4 However, drug-targeting nebulization did not affect pulmonary bioavailability compared to continuous administration. A significantly higher accumulated amount of excreted salbutamol was recovered from subjects using the targeted delivery device.
Further studies in radio-labelled aerosols will be needed to clarify the effect of targeted nebulization on drug deposition in the lungs. To conclude, drug-targeted nebulization does not change pulmonary bioavailability in healthy individuals but it does reduce the amount of drug delivered.
Clinical Trial: NCT01913184.
Authorship- a)
Study concept and design or data collection, analysis or interpretation: Gregory Reychler, Anne-Sophie Aubriot, Julien Masquelier and G. Giulio Muccioli.
- b)
Preliminary draft of the article or critical review of significant intellectual content: Gregory Reychler, G. Giulio Muccioli and Giuseppe Liistro.
- c)
Final approval of version submitted for publication: Gregory Reychler, Anne-Sophie Aubriot, Julien Masquelier, G. Giulio Muccioli and Giuseppe Liistro.
No conflict of interests.
Please cite this article as: Reychler G, Aubriot AS, Masquelier J, Muccioli GG, Liistro G. Efecto de la nebulización dirigida de fármacos en la administración pulmonar. Arch Bronconeumol. 2014;50:500–501.