Continuous Positive Airway Pressure (CPAP) is the most effective therapy for symptomatic obstructive sleep apnoea (OSA). However, uncertainty remains about the effectiveness of CPAP in improving OSA-related metabolic dysregulation. This meta-analysis of randomized controlled trials (RCTs) aimed to investigate whether CPAP, compared to other control treatments, could improve glucose or lipid metabolism in OSA patients.
MethodsRelevant articles were searched in three different databases (MEDLINE, EMBASE and Web of Science) from inception to 6th Feb 2022 through specific search terms and selection criteria.
ResultsFrom a total of 5553 articles, 31 RCTs were included. CPAP modestly improved insulin sensitivity as determined by mean fasting plasma insulin and Homeostasis Model Assessment of Insulin Resistance reduction of 1.33mU/L and 0.287, respectively. In subgroup analyses pre-diabetic/type 2 diabetic patients as well as those with sleepy OSA showed a greater response to CPAP. Regarding lipid metabolism, CPAP was associated with a mean total cholesterol reduction of 0.064mmol/L. In subgroup analyses, the benefit was higher in patients that showed more severe OSA and oxygen desaturations at the baseline sleep study as well as in younger and obese subjects. Neither glycated haemoglobin nor triglycerides, HDL- and LDL-cholesterol were reduced by CPAP.
ConclusionCPAP treatment may improve insulin sensitivity and total cholesterol levels in OSA patients but with low effect size. Our results suggest that CPAP does not substantially improve metabolic derangements in an unselected OSA population, but the effect may be higher in specific subgroups of OSA patients.
Obstructive sleep apnoea (OSA) is characterized by recurrent upper airway obstructions during sleep which lead to intermittent episodes of cessation (apnoea) or reduction (hypopnea) in airway flow despite continued inspiratory efforts. An apnoea–hypopnea index (AHI)≥5 events per hour, detected on polysomnography, defines the OSA syndrome when it is accompanied by other sleep-associated features, such as daytime or nocturnal symptoms.1
In recent years, OSA has been recognized as an independent risk factor for several cardiovascular diseases such as hypertension, coronary artery disease, heart failure, atrial fibrillation and stroke.1 Furthermore, OSA might directly impair glucose and lipids metabolism through several pathophysiological mechanisms such as intermittent hypoxia and sleep fragmentation leading to β-cells dysfunction, insulin resistance, and dyslipidemia.2–4 Moreover, the relationship between OSA and type 2 diabetes mellitus (T2DM) seems bidirectional further increasing the complexity of this issue. In fact, diabetic neuropathy might affect central respiratory control and upper airway patency leading to obstructive and central sleep-disordered breathing.5
Continuous Positive Airway Pressure (CPAP) is considered the first-line treatment for symptomatic OSA.6 Several observational studies and meta-analyses suggest that CPAP therapy can reduce OSA-related cardiovascular risk factors, especially hypertension.1,7,8 Therefore, in view of the above, it is reasonable to assume that CPAP might also improve OSA-related metabolic abnormalities. Nevertheless, a great deal of uncertainty remains on this issue, mainly because clinical studies (both observational studies and randomized controlled trials [RCTs]) revealed conflicting results. Martínez-Cerón and colleagues showed that in patients with OSA and uncontrolled T2DM, CPAP improved insulin sensitivity and glycaemic control.9 Similarly, a study limited to a population of patients with OSA and prediabetes came to the same conclusions.10 In contrast, other RCTs showed no change in lipid or glucose levels in the CPAP intervention group as compared to the control arm.11,12 Therefore, in the last few years, several systematic reviews and meta-analyses tried to shed light on these inconclusive results with a general agreement on a favourable effect of CPAP both on insulin resistance and total cholesterol (TC) levels.13–23 Only one meta-analysis showed that CPAP did not have a statistically significant effect on fasting plasma insulin (FPI).24 However, it is noteworthy that almost all studies, except for one,16 concluded that CPAP did not reduce fasting plasma glucose (FPG), glycosylated haemoglobin (HbA1c), triglycerides (TG), HDL- and LDL-cholesterol levels. Furthermore, due to the insufficient number of included studies, most of these meta-analyses were unable to perform a meaningful subgroup analysis to elucidate if the impact of CPAP could be higher in specific subsets of patients. Furthermore, all of them were restricted to a predetermined patient's population (i.e., nondiabetic/prediabetic/diabetic subjects) and limited to the evaluation of either glucose-insulin or lipids levels.
Hence, we performed a larger systematic review and meta-analysis in unselected OSA patients to explore whether CPAP could contribute to restore glucose and lipid homeostasis in these subjects. Furthermore, the present study tried to identify if there were specific subgroups or phenotypes of patients that could predict a better response to CPAP.
Study design and methodsThe systematic review and meta-analysis were performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.25 The meta-analysis was conducted in conformity with the general recommendations of the Cochrane Handbook for Systematic Reviews of Interventions25,26 and was registered at PROSPERO with identifier number CRD42018093961.
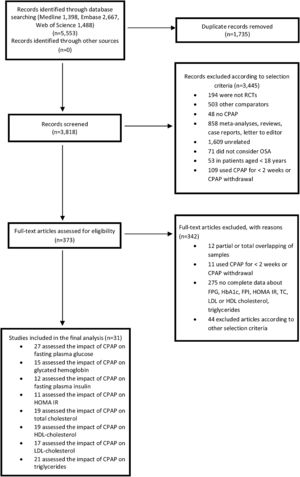
Literature search and selection of the trialsA systematic computerized search of three different databases (MEDLINE, EMBASE and Web of Science) was performed, from inception to 6th Feb 2022, to detect all RCTs that evaluated the impact of CPAP treatment on glucose-insulin and lipid levels in patients suffering from OSA. The search algorithm combined the categories for “OSAS,” “CPAP therapy,” and “randomized controlled trials” by the Boolean operator AND (for details please see the supplementary material section “literature search and selection of the trials”).
Studies were selected for inclusion if all the following criteria were met:
- 1.
Adult patients (aged≥18 years) suffering from OSA, defined by an AHI≥5 events per hour of sleep or with more than 7.5 desaturations dips>4% per hour of sleep27;
- 2.
A diagnosis of OSA obtained by full polysomnography or cardiorespiratory polygraphy or home sleep apnea tests;
- 3.
RCTs that compared CPAP therapy with either sham-CPAP (when CPAP is used at subtherapeutic pressure), oral placebo (e.g. oral tablets) or conservative measures such as standard care and counselling;
- 4.
RCTs had to report for both groups of interest the following information for at least one of the considered outcomes: mean changes (follow-up – baseline) and between-patient variations (standard error [SE] or standard deviation [SD] or 95% confidence interval [95% CI]) or sufficient data to their calculation. The outcomes of interest were: (i) FPG, (ii) HbA1c, (iii) FPI, (iv) Homeostasis model assessment of insulin resistance (HOMA-IR), (v) TC, (vi) HDL-cholesterol, (vii) LDL-cholesterol, (viii) TG, as indices of glucose-insulin and/or lipid metabolism;
- 5.
Treatment had to last at least two weeks.
A diagnosis of central apnoea syndrome (defined as a cessation of airway flow without inspiratory effort greater than 50% of total apnoeic episodes) was considered as an exclusion criterium. We also excluded withdrawal studies and studies that compared CPAP treatment with weight loss achieved through either structured physical activity or nutritional counselling that were considered as active therapies.
Two independent investigators (FC and MP) retrieved all relevant publication according to the above-mentioned criteria. In the event of disagreements, a final consensus was reach after discussion with CF.
Data extractionTwo independent investigators (FC and AG) retrieved the data of interest from the included studies. Inconsistencies were resolved after discussion with CF. For details, please see the supplementary material, section “Data extraction”.
Quality assessmentThe methodological quality of included study was evaluated using the JADAD scale31 and through a self-made score already used by our group.7 For details, please see please see the supplementary material, section “Quality assessment”.
Statistical analysisThe pooled differences of changes (FU-baseline) between CPAP and controls groups were the main measure of interest (Δ=((FU−baseline)CPAP−(FU−baseline)Control). When the SDs of the changes were not available we calculated them by the following formula SD=square root [(SDCPAP)2+(SDcontrol)2−(2*rho* SDCPAP*SDControl)], where the value of correlation (rho) was fixed at 0.5. The pooled mean differences and corresponding 95% confidence interval of FPG, FPI, HbA1c, HOMA-IR, TC, LDL-cholesterol was calculated implementing Der Simonian and Laird random-effects method.28 All tests were 2-sided, and p-values<0.05 were considered statistically significant. All statistical analyses were performed with Comprehensive Meta Analysis, version 2.2.064 (Biostat, Inc) software. For details about methods used to assess heterogeneity and bias across studies as well as on how subgroups analyses were performed, please see the supplementary material, section “Statistical analysis”.
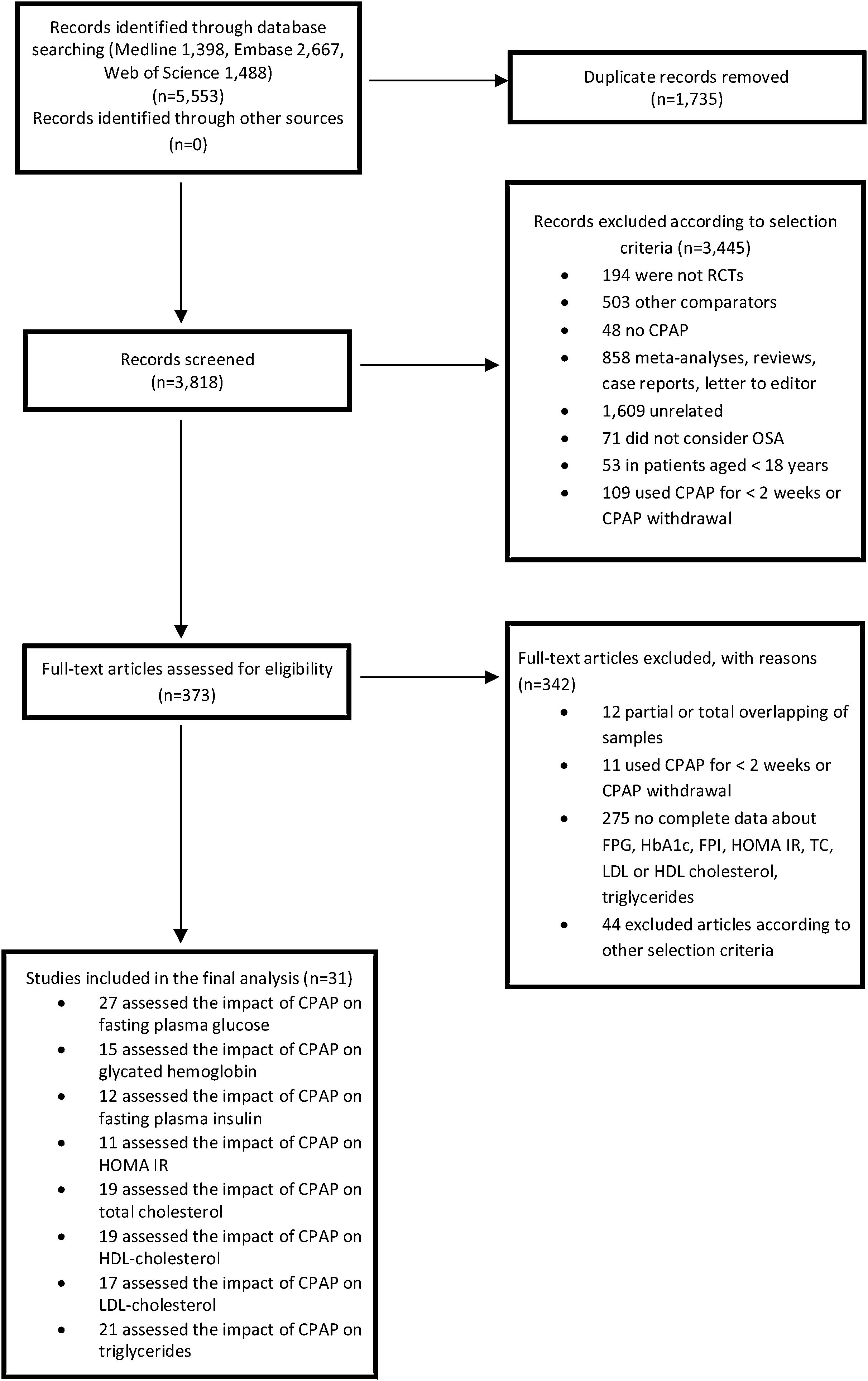
ResultsStudy selectionFrom a total of 5553 articles, 31 RCTs met the inclusion criteria and were included in the meta-analysis. The k statistic indicated a strong agreement between the extracting investigators (kappa=0.83±0.04; p<0.001). Fig. 1 provides details of the results of literature search. The characteristics of the primary studies are summarized in Table S1. In brief, the baseline mean age and body mass index (BMI) were 55.03 years and 32.20kg/m2 respectively, whereas the 75.77% of the included patients were males. The median follow-up duration was 12 weeks (minimum to maximum: 2–48 weeks) and the median CPAP adherence was 4.05h/night (minimum to maximum: 2.22–8.00h/night).
Impact of CPAP treatment on glucose-insulin levelsTwenty-seven trials for a total of 2453 patients provided data of FPG levels before and after treatment with CPAP and controls. Random-effects meta-analysis showed that CPAP therapy was associated with a decrease in FPG levels (MD=−0.057mmol/L, 95% CI=−0.107 to −0.008, I2=0.0%, Fig. S1). The funnel plot (Fig. S6a) showed low risk of publication bias (p=0.8 in modified Egger test) whereas the influence analysis revealed that the significant effect was driven by a single study conducted in a population of neither diabetic nor prediabetic patients30 and after its removal the statistical significance disappeared (Fig. S8a).
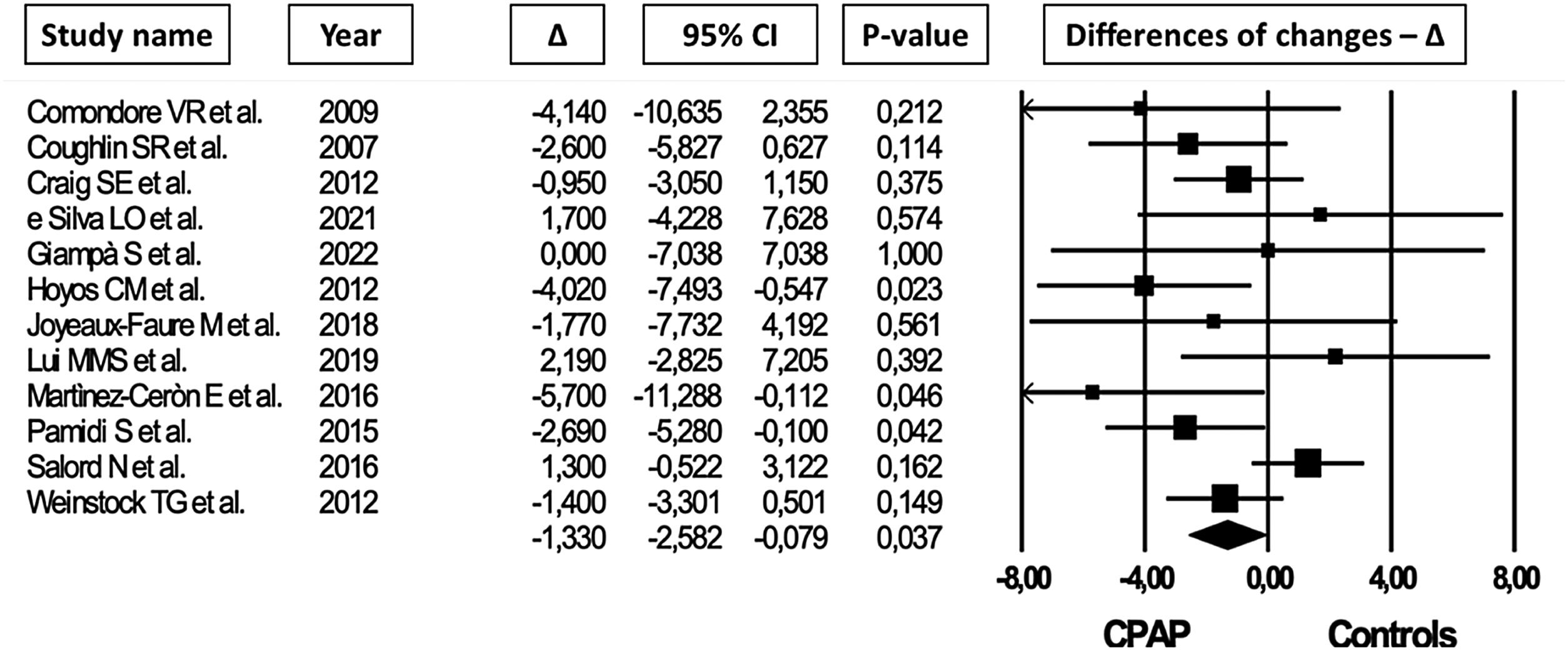
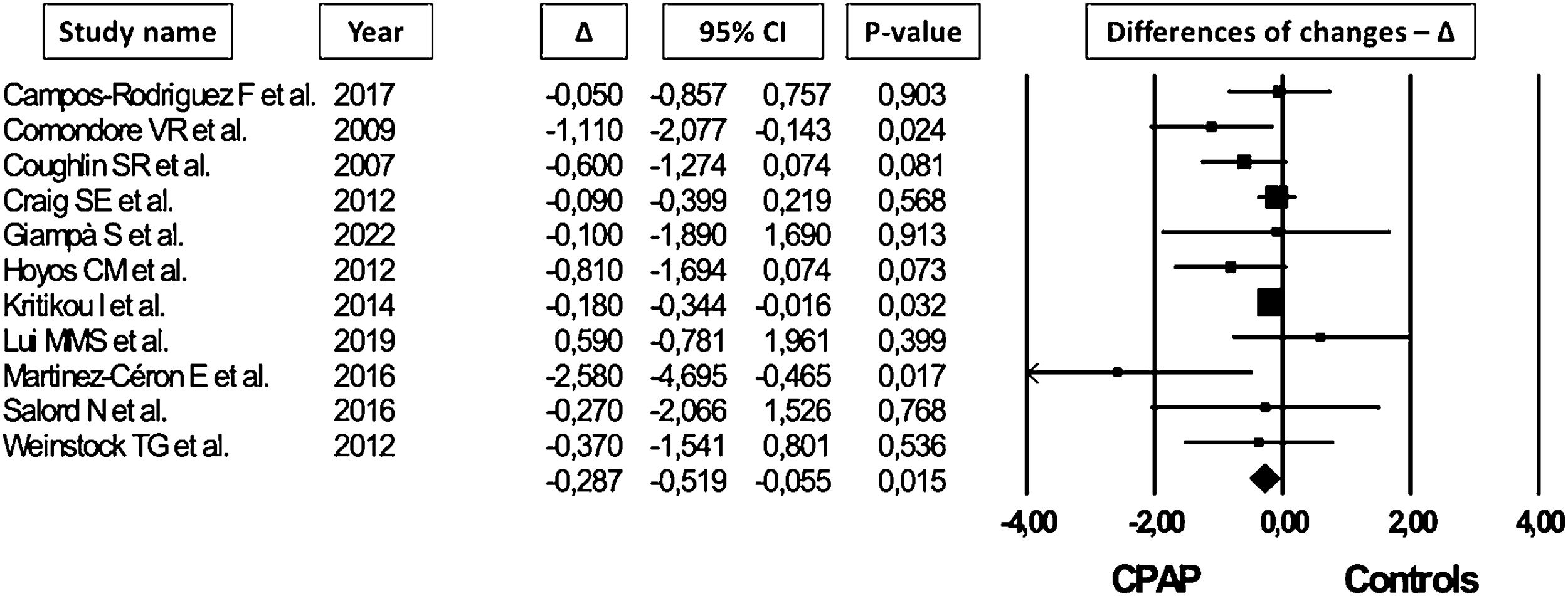
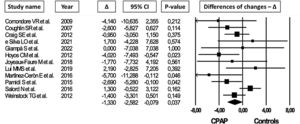
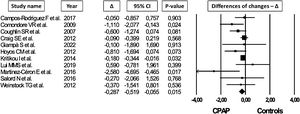
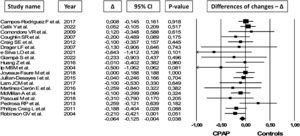
Data about FPI levels and HOMA IR were available in 12 and 11 trials for a total of 832 and 1058 OSA patients, respectively. In random-effect meta-analysis, CPAP reduced both FPI (MD=−1.330mU/L, 95% CI=−2.582 to −0.079, I2=38.4, Fig. 2) and HOMA IR (MD=−0.287, 95% CI=−0.519 to −0.055, I2=25.7, Fig. 3) without significant risk of publication bias (funnel plots: Fig. S6b-c; p=0.6 and p=0.2 in modified Egger test for FPI and HOMA IR, respectively).
CPAP therapy did not modify HbA1c levels (MD=−0.010%, 95% CI=−0.053 to 0.034, I2=15.1%, Fig. S2, 15 trials for a total of 1783 patients) even omitting the study of Comondore and colleagues,29 the only one in which follow-up duration (4 weeks) would not allow to observe a meaningful change in HbA1c levels. The funnel plot (Fig. S6d) showed no risk of publication bias (p=0.7 in modified Egger test).
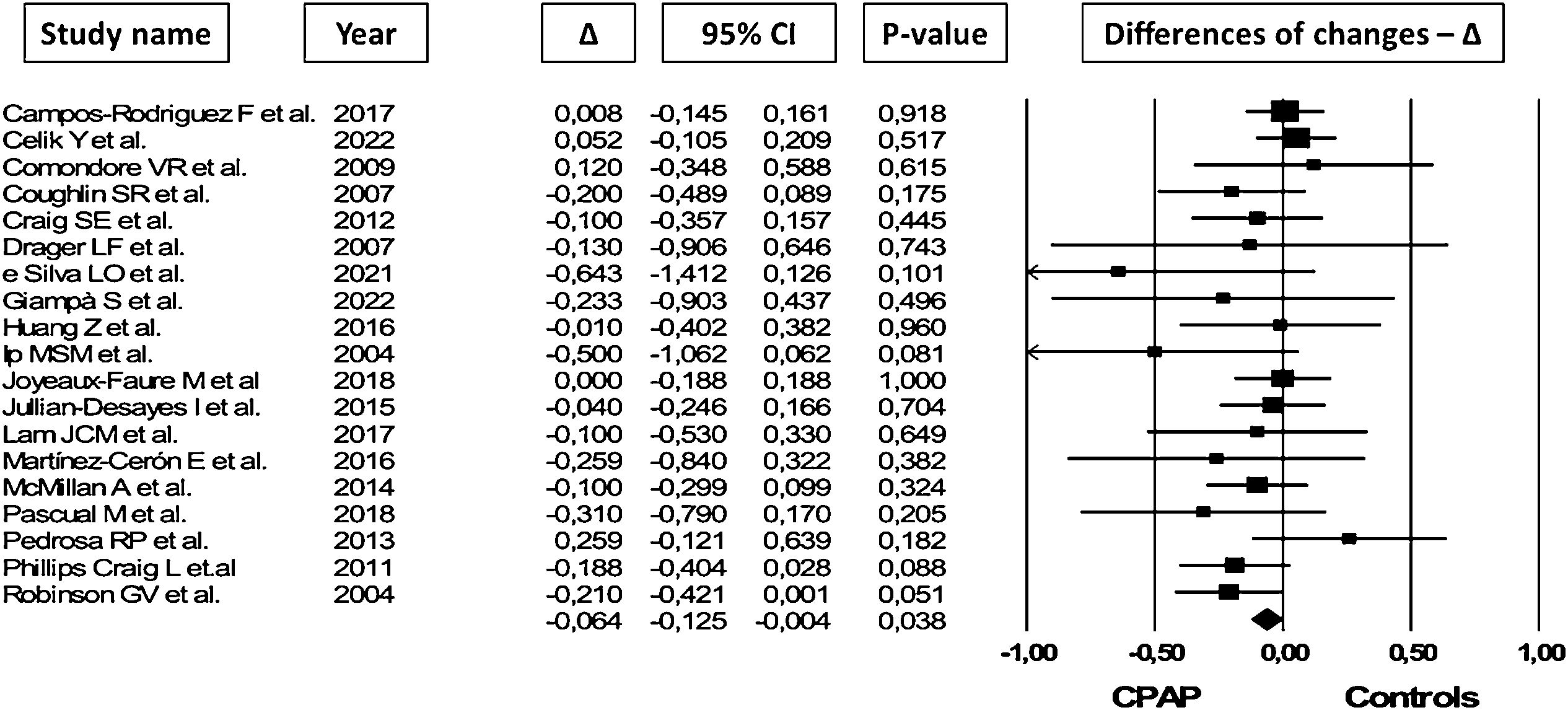
Impact of CPAP treatment on lipids levelsData about TC levels were available in 19 studies for a total of 1943 OSA patients. In random-effects meta-analysis CPAP treatment was associated with a mean TC levels reduction of 0.064mmol/L (95% CI=−0.125 to −0.004, I2=0.0%, Fig. 4). Funnel plot inspection and the other tests suggested a possible risk of publication bias (Fig. S6e; p=0.06 in modified Egger test). The trim and fill analysis indicated 5 potential missing trials favouring control therapies and after symmetrically filling the funnel plot the statistical significance disappeared (MD=−0.047mmol/L, 95% CI=−0.111 to 0.014, Fig. S7).
CPAP treatment did not increase HDL-cholesterol (MD=−0.001mmol/L, 95% CI=−0.018 to 0.017, I2=0.0%, Fig. S3, 19 studies for 1750 patients) or reduced LDL-cholesterol (MD=−0.025mmol/L, 95% CI=−0.082 to 0.031, I2=0.0%, Fig. S4, 17 studies for 1621 patients), and TG (MD=−0.013mmol/L, 95% CI=−0.063 to 0.036, I2=0.0%, Fig. S5, 21 studies for 1954 patients) levels as compared with other treatments. Funnel plots for HDL-cholesterol, LDL-cholesterol and TG did not show any significant asymmetry (Figs. S6f–h).
Subgroup analysisSleepy patients (Epworth Sleepiness Scale [ESS]≥10) benefited the most from CPAP therapy in terms of FPI levels reduction (Fig. S9a) whereas the favourable effects of CPAP on HOMA IR levels were maintained only in patients with pre-diabetes or T2DM (Fig. S9b), albeit the two groups were not significantly different (p=0.4 for the comparison between ESS<10 vs ESS≥10 and p=0.1 for the comparison between pre-diabetes/T2DM vs. nondiabetic patients). Regarding OSA severity, the results remained significant in patients with an AHI≥30events/h; however, due to the inadequate number of studies with non-severe OSA (only 2 and 1 for FPI and HOMA IR, respectively) the subgroups analysis was not performed. No differences in the treatment effect were observed dividing the studies according to the following baseline characteristics: patients’ age or BMI, median FPI or HOMA IR baseline levels, CPAP usage (<4h/nights vs ≥4h/nights), length of CPAP treatments (<12 weeks vs ≥12 weeks) and methodological quality of the included trial (JADAD<4 vs ≥4; self-made score<5 vs ≥5). The subgroups analysis according to the oxygen saturation parameters (SpO2-nadir<77% vs ≥77%; time spent with SpO2<90% of total sleep time<7% vs ≥7%) was not performed to the insufficient number of studies.
The effect of CPAP treatment in term of TC levels reduction was maintained in patients with severe OSA (AHI≥30events/h) (Fig. S9c) and in those who presented more severe nocturnal hypoxia at baseline (SpO2-nadir<77%) (Fig. S9d), although there was no difference between the groups (p=0.3 for the comparison between AHI<30 events/h vs AHI≥30 events/h and p=0.2 for the comparison between SpO2-nadir≤77% vs SpO2-nadir>77%). Furthermore, dividing the primary studies according to the baseline median age and BMI (median age=55.5; median BMI=32kg/m2), younger patients (Fig. S9e) (p=0.005 for the comparison between age<55.5 years vs age≥55.5 years) and those with higher BMI (Fig. S9f) (p=0.05 for the comparison between BMI<32kg/m2 vs BMI≥32kg/m2) showed a greater TC levels reduction in response to CPAP treatment. No differences in the treatment effect were observed dividing the studies according to the following baseline characteristics: median TC levels, CPAP usage (<4h/nights vs ≥4h/nights), length of CPAP treatments (<12 weeks vs ≥12 weeks), time spent with SpO2<90% of total sleep time (<7% vs ≥7%) and methodological quality of the included trial (JADAD<4 vs ≥4; self-made score<5 vs ≥5).
Stratified analyses, according to the above-mentioned criteria, for HbA1c, HLD-cholesterol, LDL-cholesterol or TG levels reduction yielded no significant findings.
DiscussionThis meta-analysis showed a statistically significant positive effect of CPAP treatment on FPG levels, insulin sensitivity and TC levels in an unselected OSA population but with a very low effect size. The benefits of CPAP on insulin resistance were greater in patients with pre-diabetes or T2DM and in those with sleepy OSA. Regarding TC, more severe OSA and severe baseline oxygen desaturations, as well as younger age and obesity, were associated with a greater CPAP effectiveness.
The finding of a reduction in FPG levels after CPAP should be interpreted with caution. In fact, the effect size was extremely low questioning, apart from the borderline statistical significance, its real clinical relevance. Furthermore, and probably more importantly, the decrease in FPG levels was driven by a single study and not maintained in the sensitivity analysis after its removal.
Regarding insulin metabolism, even the reduction of FPI and HOMA IR levels needs to be carefully interpreted given the small effect size. However, the subgroups analysis provided some interesting insights. The finding that patients with pre-diabetes or T2DM benefited most from CPAP could be of clinically relevance since several longitudinal studies demonstrated that OSA was significantly associated with worsening T2DM during follow-up.30,31 We can speculate that CPAP might contribute to reduce insulin resistance and may improve the metabolic control of prediabetic and diabetic patients with long-term potential to reduce the burden of micro and macrovascular complications. However, further studies are needed to confirm this hypothesis. We observed a trend towards a greater improvement in insulin resistance in sleepy patients and in those with greater AHI at baseline, albeit we were unable to perform a meaningful comparison according to OSA severity due to the lack of primary studies that enrolled patients with AHI<30 events/h. However, these findings might be of interest since they support the well-known association between OSA severity and metabolic derangements.2,5
Although it is reasonable to assume that CPAP could improve glycemic status in OSA patients, HbA1c levels were not significantly reduced by CPAP treatment both in the overall and stratified analysis. These findings seem in contradiction to data about insulin sensitivity but can also reflect the high clinical heterogeneity in terms of the selected populations, adherence to CPAP therapy, follow-up duration, sample size. Furthermore, it is very difficult to interpret the results of these studies due to the confounding effect and influence of hypoglycemic medication (both insulin and oral drugs) in T2DM patients. Martínez-Cerón and colleagues showed that CPAP treatment was effective in reducing HbA1c, as compared to placebo, in a population of OSA patients with uncontrolled T2DM on stable antidiabetic therapy.32 In another study, in the per-protocol analysis, after excluding patients who changed their anti-diabetic drugs, CPAP therapy was effective in improving HbA1c by 0.4% as compared to the control group.33 By the contrary, in a third study, while there were no changes in anti-diabetic medications during the trial, the number of patients using insulin at baseline was higher in the control group. When the analysis was limited to non-insulin users, patients treated with CPAP had a significant increase in HbA1c with respect to patients in the control arm.34
Regarding lipids metabolism, the significant difference in total cholesterol levels between CPAP-treated patients and controls should be critically considered due to the low effect size and the result of the trim and fill analysis. Anyhow, in the stratified analysis, we found that a more severe oxygen desaturations, as well as severe OSA, predicted a better response to CPAP treatment. In fact, a minimum nocturnal SpO2<77% and an AHI≥30events/h were associated with a greater TC reduction in the CPAP intervention group. This finding is consistent with the currently available evidence that identifies chronic intermittent hypoxia as one of the cardinal mechanisms behind the metabolic abnormalities observed in OSA patients2–5 and is in line with our recent meta-analysis about the impact of CPAP on blood pressure.7 Of note, we observed that other predictors of TC reduction in treated OSA were younger age and greater BMI of the enrolled patients. These findings are coherent with the recent hypothesis that OSA has a different clinical expression between young and middle-age or older individuals. In fact, in older patients, it seems that OSA is not so heavily associated with cardiometabolic comorbidities and metabolic syndrome contrary to what observed in younger ones.35 This could be of considerable clinical relevance in terms of cardiovascular prevention. Lowering TC levels in patients of younger age and greater BMI, CPAP might reduce the burden of cardiovascular disease in high-risk OSA patients. However, further studies are needed to confirm this hypothesis. By the contrary, LDL-cholesterol and triglycerides levels were not reduced by CPAP questioning the real clinical effect of this treatment on lipids metabolism. Again, the high clinical heterogeneity in terms of CPAP compliance, study design and follow-up duration of available RCTs may at least partially explain these apparently contradictory results. Furthermore, dyslipidemia could be challenging to assess as an outcome since depending on several factors such as feeding state, dietary habits, physical activities, medications (insulin, lipid-lowering drugs, alpha- and beta-blockers), sleep quality and duration.3 Notably, there was high heterogeneity in terms of statin use between the primary studies blurring the interpretation of the results of the meta-analysis. Some trials did not allow chronic use of any medication,36–38 whereas others enrolled OSA patients in secondary cardiovascular prevention and consider the effect of CPAP treatment on top of that of lipid-lowering drugs.39,40
To the best of our knowledge, this is the first and largest meta-analysis that explored the effects of CPAP treatment both on glucose-insulin and lipid levels; moreover, it was not restricted, unlike previous ones,13-16,24 to non-diabetic or prediabetic/type 2 diabetic populations further enhancing its comprehensiveness. As opposed to several previous meta-analyses,21 the decision to include only RCTs adds to data quality and results reliability. Finally, due to the adequate number of included studies we could perform a meaningful stratified analysis to investigate which subgroups or phenotypes of patients predict a better response to CPAP treatment.
The present meta-analysis has several limitations. Although systematic research was performed through a highly sensitive search strategy, we cannot guarantee that all the potentially eligible primary studies were included. Some of the applied inclusion or exclusion criteria might be questionable; for example, the decision to include only RCTs with a minimum follow-up of 2 weeks could be interpreted as to limit our study inclusion. However, the decision was taken believing that there is a minimum time required to detect a stable positive metabolic effect in response to CPAP treatment. Finally, due to the low number of primary studies we could compare nondiabetic to prediabetic along with diabetic patients. The choice may be controversial because of the high clinical heterogeneity of these populations.
In conclusion, our results align with the currently available literature suggesting that CPAP may exert a favourable effect on insulin homeostasis and TC levels in OSA patients, but with a very low effect size suggesting modest clinical significance. Subgroup analyses showed that OSA severity and nocturnal hypoxaemia could be predictors of a more favourable response to CPAP treatment. Furthermore, the effect of CPAP could partially depend on the magnitude of the metabolic derangements as indicated by the fact that prediabetic/type 2 diabetic patients benefited the most in terms of insulin resistance reduction. However, the metabolic consequences of OSA treatment, especially on lipid metabolism, remains poorly characterized. Further well-designed RCTs with adequate CPAP adherence, and especially on at-risk populations, such prediabetic/diabetic and dyslipidemic patients or in those with greater hypoxaemic burden, severe and/or sleepy OSA, are needed to clearly establish whether CPAP could have a clinically relevant and independent effect on metabolic derangements observed in these patients.
FundingNone to declare.
Conflict of interestNone to declare.