Malignant pleural effusion (MPE) is encountered in fifteen percent of patients with cancer1 and frequently causes breathlessness that recurs after aspiration which necessitates more definitive treatment such as indwelling pleural catheter insertion or pleurodesis.2 Talc is the agent used for pleurodesis in the UK2 due to its superior efficacy compared to other agents1 with success rates reported from large trials ranging between 75 and 80%.3,4 About one quarter of the patients receiving talc will require further procedures. Some parameters, such as pleural fluid pH and adenosine deaminase5,6 have been correlated with pleurodesis outcome but these results have not been replicated in other studies and their overall clinical usefulness remains questionable.
Thoracic ultrasound (TUS) is an essential tool in the evaluation of pleural effusion.7 Echogenic swirling (ES), a finding which describes multiple floating echogenic particles inside an effusion (supplementary video), is seen on TUS examination of some patients with MPE and has been hypothesised to be related to enhanced intrapleural fibrinolytic activity.8 Effusions of MPE patients who failed pleurodesis were shown to have higher levels of fibrinolytic enzymes9 and MPE samples obtained following intapleural application of sclerosants tended to exhibit more sustained elevation in levels of fibrin degradation products in patients who failed the procedure.10,11 This study aimed to assess whether an ultrasound parameter available prior to intervention (ES) is associated with reduced rates of pleurodesis success.
Data on patients who underwent thoracoscopic talc poudrage or talc slurry instillation via chest drain for the management of MPE at the authors’ Pleural Unit between 2016 and 2017 were collected. Baseline clinical details and pleural fluid biochemistry results were retrieved. Recorded TUS clips of pre-procedure effusions were assessed for the presence of ES.
Pleurodesis failure was defined as the need for another therapeutic procedure within the first 3 months after pleurodesis.
Patients were excluded if there were no assessable TUS clips on the database, post-drainage X-ray showed any degree of trapped lung, or data on pleurodesis outcome was not available.
Overall rate of pleurodesis success as well as according to pleural fluid echogenicity was presented. All factors that are hypothesised to affect pleurodesis were entered into a multiple logistic regression analysis to identify variables associated with pleurodesis failure.
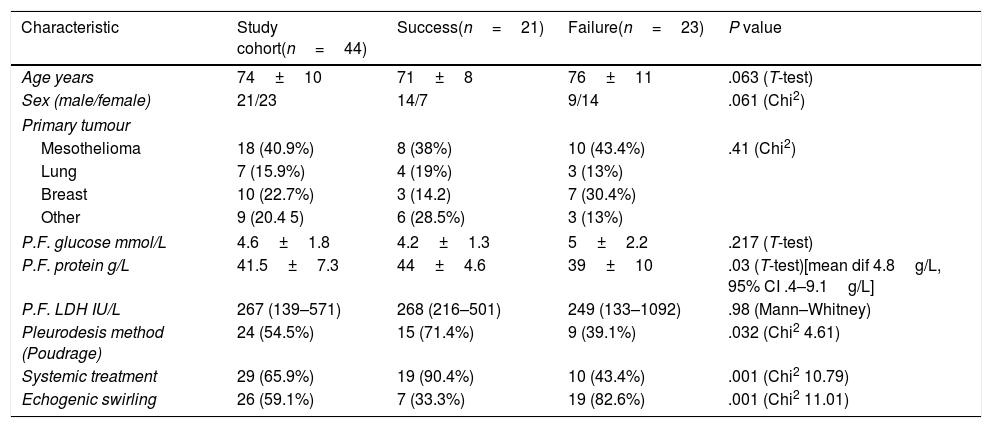
Sixty nine patients underwent pleurodesis for MPE in the period of study. Forty four patients were included, with the remaining excluded due to the absence of assessable TUS clips (18 patients), trapped lung (2 patients), or death prior to follow up visit (5 patients). Table 1 shows the clinical and pleural fluid characteristics of the studied patients. Pleurodesis failure was noted in 23/44 (52.2%) of the study cohort. Mean pleural fluid protein was higher in patients who failed pleurodesis (mean dif 4.8g/L, 95% CI 0.4–9.1g/L). Regarding the relation between pleurodesis outcome and the presence of ES, ES was found in 7/21 (33.3%) with successful pleurodesis and in 19/23 (82.6%) with failed pleurodesis (Chi2=11.01, 1df, p=0.001).
Baseline characteristics and pleural fluid composition of the study cohort and according to pleurodesis outcome. Data for continuous variables is presented as mean+SD or median (IQR) and for discrete variables as frequency (per cent).
| Characteristic | Study cohort(n=44) | Success(n=21) | Failure(n=23) | P value |
|---|---|---|---|---|
| Age years | 74±10 | 71±8 | 76±11 | .063 (T-test) |
| Sex (male/female) | 21/23 | 14/7 | 9/14 | .061 (Chi2) |
| Primary tumour | ||||
| Mesothelioma | 18 (40.9%) | 8 (38%) | 10 (43.4%) | .41 (Chi2) |
| Lung | 7 (15.9%) | 4 (19%) | 3 (13%) | |
| Breast | 10 (22.7%) | 3 (14.2) | 7 (30.4%) | |
| Other | 9 (20.4 5) | 6 (28.5%) | 3 (13%) | |
| P.F. glucose mmol/L | 4.6±1.8 | 4.2±1.3 | 5±2.2 | .217 (T-test) |
| P.F. protein g/L | 41.5±7.3 | 44±4.6 | 39±10 | .03 (T-test)[mean dif 4.8g/L, 95% CI .4–9.1g/L] |
| P.F. LDH IU/L | 267 (139–571) | 268 (216–501) | 249 (133–1092) | .98 (Mann–Whitney) |
| Pleurodesis method (Poudrage) | 24 (54.5%) | 15 (71.4%) | 9 (39.1%) | .032 (Chi2 4.61) |
| Systemic treatment | 29 (65.9%) | 19 (90.4%) | 10 (43.4%) | .001 (Chi2 10.79) |
| Echogenic swirling | 26 (59.1%) | 7 (33.3%) | 19 (82.6%) | .001 (Chi2 11.01) |
IQR: interquartile range; SD: standard deviation.
The levels of protein, LDH and glucose did not differ significantly between effusions exhibiting ES and those without this sign. Mean protein was 41±8g/L in effusion with ES vs. 41±6g/L in those without ES. Median (IQR) LDH was 251 (11–885) IU/L with ES vs. 268 (166–348) in effusions without ES and median glucose was 4.4mmol/L (3.5–6.1) vs. 4.2 (3.3–5.7) in effusion without ES.
Regarding the primary cause of MPE, 12/18 (66.6%) cases with mesothelioma had ES on TUS examination, while ES was seen in 4/7 (57.1%) patients with lung cancer and in 6/10 (60%) patients with breast cancer (Chi2=1.24, 2df, p=0.74).
Multiple logistic regression analysis on pleurodesis success as the dependent variable included the presence of ES together with institution of systemic cancer therapy, talc poudrage as the method of pleurodesis and pleural fluid protein level as the independent variables. The factors independently associated with pleurodesis success according to regression analysis were the presence of ES on US (odds ratio 0.016, 95% CI 0.001–0.310, p=0.006) and lack of systemic therapy (odds ratio 0.014, 95%CI 0.001–0.304, p=0.007).
This study suggests that ES seen on TUS examination of patients with MPE may be associated with lower rates of success of pleurodesis and this is independent of other parameters such as the presence of systemic therapy.
MPEs are characterised by enhanced inflammatory activity and fibrin deposition,12 coupled with high levels of fibrinolytic enzymes and fibrin degradation products.12 It is thought that the echogenic material seen floating on TUS examination of MPE could be the broken down fibrinous material from the aforementioned processes.8 The association of ES with lower success rates of pleurodesis could be linked to the previously noted high levels of fibrinolytic enzymes such as u-PA in MPE patients who failed pleurodesis.9
The remarkably lower rates of pleurodesis success in cases with ES might thus be explained by failure of pleural fibrosis and symphysis that characterises pleurodesis and which could be antagonised by high fibrinolytic activity in such effusions. Indeed higher levels of D-dimer (a marker of fibrinolysis) have been noted in MPE with failed pleurodesis in comparison to successful pleurodesis.10,11 Notably, the chemical composition of effusions showing ES was not different from that of effusions not showing ES.
The presence of ES was judged in a binary fashion which could be an oversimplification of a variable that can manifest with various degrees of intensity. It is not possible using current ultrasound machines to objectively quantify the degree of echogenicity of an effusion. However, the concept of ‘echogenicity index’ has been used to quantify the degree of echogenicity of structures such as thyroid nodules13 and renal parenchyma14 using softwares that compare the echogenicity of a structure in question to adjacent ‘reference’ organs to gain clinically relevant information.
The decision to exclude patients who had trapped lung (which is related to the extent of visceral pleural involvement) from the analysis was intended to remove confounding effect of heavier pleural tumour burden on pleurodesis failure. The definition of pleurodesis failure was based on objective criteria that mirror the definition used in large clinical trials of pleurodesis3 in order to ensure the robustness of the data obtained.
This study has several limitations; we found a higher than usual failure rate of pleurodesis (52%). The studied cohort has a high proportion of mesothelioma cases which is known to respond less favourably to pleurodesis15 and reflects differences that are seen often between data derived from trials and that seen in the ‘real-world’. Despite the fact that this study involved a relatively small number of patients, there was strong signal to support a link between pleural fluid echogenicity and pleurodesis outcome.
The proposed TUS-based observation of ES as a tool for outcome prediction of pleurodesis is a potentially useful non-invasive and easy to interpret tool, which is importantly available to the clinician prior to any decision on intervention. This may therefore help towards better customisation of management according to patients’ characteristics. Our results require prospective validation in a larger patient cohort and correlation with other variables such as pleural fluid cytological positivity and the presence of pleural thickening. The role that a pro-fibrotic/fibrinolytic balance may play in determining pleural fluid echogenicity and pleurodesis outcome and the usefulness of quantifying pleural fluid echogenicity are areas of potential future research.
This work uses data provided by patients and collected by the NHS as part of their care and support, and is therefore considered an audit not requiring ethical approval.