Hemoptysis is the expectoration of blood from the tracheobronchial tree. It is commonly caused by bronchiectasis, chronic bronchitis, and lung cancer. The expectorated blood usually originates from the bronchial arteries. When hemoptysis is suspected, it must be confirmed and classified according to severity, and the origin and cause of the bleeding determined. Lateral and AP chest X-ray is the first study, although a normal chest X-ray does not rule out the possibility of malignancy or other underlying pathology. Multidetector computed tomography (MDCT) must be performed in all patients with frank hemoptysis, hemoptoic sputum, suspicion of bronchiectasis or risk factors for lung cancer, and in those with signs of pathology on chest X-ray. MDCT angiography has replaced arteriography in identifying the arteries that are the source of bleeding. MDCT angiography is a non-invasive imaging technique that can pinpoint the presence, origin, number and course of the systemic thoracic (bronchial and non-bronchial) and pulmonary arterial sources of bleeding. Endovascular embolization is the safest and most effective method of managing bleeding in massive or recurrent hemoptysis. Embolization is indicated in all patients with life-threatening or recurrent hemoptysis in whom MDCT angiography shows artery disease. Flexible bronchoscopy plays a pivotal role in the diagnosis of hemoptysis in patients with hemoptoic sputum or frank hemoptysis. The procedure can be performed rapidly at the bedside (intensive care unit); it can be used for immediate control of bleeding, and is also effective in locating the source of the hemorrhage. Flexible bronchoscopy is the first-line procedure of choice in hemodynamically unstable patients with life-threatening hemoptysis, in whom control of bleeding is of vital importance. In these cases, surgery is associated with an extremely high mortality rate, and is currently only indicated when bleeding is secondary to surgery and its source can be accurately and reliably located.
La hemoptisis es la expectoración de sangre proveniente del árbol traqueobronquial. Las enfermedades que más frecuentemente la originan son las bronquiectasias, la bronquitis crónica y el carcinoma broncogénico. Las arterias bronquiales son el origen de la mayoría de las hemoptisis. Ante un paciente con sospecha de hemoptisis se debe confirmar su existencia, establecer su gravedad, localizar el origen y determinar su causa. La radiografía de tórax posteroanterior y lateral es la primera prueba de imagen que debe realizarse, aunque la existencia de una radiografía de tórax normal no excluye la posibilidad de malignidad u otra patología de base. Debe realizarse TC multidetector (TCMD) de tórax en todos los pacientes con hemoptisis franca, en los que presentan esputo hemoptoico y sospecha de bronquiectasias o factores de riesgo de carcinoma broncogénico, y en los que tienen radiografía de tórax patológica. La angio-TCMD ha sustituido a la arteriografía como método diagnóstico de las arterias que son fuente de sangrado en las hemoptisis. La angio-TCMD es una técnica de imagen no invasiva que identifica correctamente la presencia, el origen, el número y el trayecto de las arterias sistémicas torácicas, bronquiales y no bronquiales, y de las arterias pulmonares que pueden ser fuente del sangrado. El tratamiento más seguro y eficaz para detener el sangrado en la mayoría de los casos de hemoptisis masiva o recurrente es la embolización endovascular. La embolización está indicada en todos los pacientes con hemoptisis amenazante o recurrente en los que se detectan arterias patológicas en la angio-TCMD. La broncoscopia flexible juega un papel primordial en el diagnóstico de la hemoptisis, tanto de la expectoración hemoptoica como de la hemoptisis franca. Puede ser realizada rápidamente en la cama del paciente (UCI) y, además de su utilidad en el control inmediato de la hemorragia, tiene una alta rentabilidad en la localización del sangrado. La broncoscopia flexible es el procedimiento inicial de elección en pacientes con hemoptisis amenazante e inestabilidad hemodinámica, donde el control de la hemorragia es vital. La cirugía en estos casos tiene una tasa de mortalidad muy alta, por lo que la indicación actual de cirugía en la hemoptisis amenazante está reservada para aquellas situaciones en las que la causa de la misma sea tributaria de tratamiento quirúrgico y haya una localización concreta y fiable del origen de la hemorragia.
Since the 1994 publication of the SEPAR guidelines1 on the management of life-threatening hemoptysis, significant advances have been made in diagnostic and therapeutic techniques, necessitating an update of the diagnostic and therapeutic recommendations, not only for life-threatening hemoptysis but also for other less critical but equally important situations. The prognosis of patients with hemoptysis has improved substantially in recent years, thanks to improved thoracic radiology and bronchoscopic techniques and the implementation of multidisciplinary management.
Patients with hemoptysis must be fully evaluated according to a clinical protocol to determine the most appropriate diagnostic and therapeutic procedures. These SEPAR guidelines outline the definition, classification and etiology, diagnosis (initial evaluation, bronchoscopy and radiology), treatment (general measures, therapeutic bronchoscopy, arterial embolization and surgery) of hemoptysis and the management of special situations, such as life-threatening hemoptysis. Life-threatening hemoptysis is one of the emergencies most feared by pulmonologists. Due to its acute, high risk nature, it has been the subject of very few prospective diagnostic and therapeutic studies, and therefore the quality of the scientific evidence used to establish recommendations for management is generally low.
The GRADE system2 has been adopted to classify the strength of recommendations based on expected risk/benefit ratios (strong 1, weak 2); the quality of scientific evidence is defined as high (A), moderate (B), low (C), or very low (D).
Definition and EtiologyHemoptysis is the expectoration of blood from the tracheobronchial tree. It has multiple causes and ranges in severity from blood-streaked sputum, gross hemoptysis (expectoration of blood only), and massive hemoptysis (expectoration of large amounts of fresh blood).
Massive hemoptysis has been defined in the literature by several different criteria, ranging from 100ml to 600ml of blood over wide-ranging periods of time. These variations in definition are compounded by the difficulty in quantifying the amount of blood expectorated, which is usually overestimated by patients. However, underestimation is also an issue, as part of the blood may be retained in the tracheobronchial tree.
We prefer to use the term life-threatening hemoptysis, defined as hemoptysis which jeopardizes the patient's life; the risk is determined by the total volume and speed of the bleeding, and the patient's cardiopulmonary reserve.1 Risk factors to be taken into account include the volume of hemoptysis (greater than 100ml) and the presence of airway obstruction, respiratory failure or hemodynamic instability.3
Vascular Origin of HemoptysisThe lung is supplied by blood from 2 systems: the pulmonary arteries and the bronchial arteries. The pulmonary arteries form a low pressure system through which the cardiac output circulates, and are responsible for gas exchange. Bronchial arteries are part of the systemic circulation and carry blood at a higher pressure and a much lower flow rate; these vessels are responsible for the irrigation of the bronchi and the visceral pleura. Even though they contribute less to the pulmonary blood flow, the bronchial arteries are the source of most hemoptysis, although non-bronchial systemic arteries can also be involved. A much lower percentage of bleeding originates in the pulmonary arteries and in the pulmonary microcirculation.4
The vessels in the bronchial network that cause bleeding are usually newly formed, generally secondary to inflammatory disease (bronchiectasis, pulmonary abscess, tuberculosis). The walls of these vessels are surrounded by smooth muscle fiber that contracts due to both physical and pharmacological stimuli. Arterial embolization is an effective method of eliminating this neovascularization. However, vasospasms in the pulmonary arterial network are not as strong as those occurring in the bronchial vessels. This is because the walls of these vessels are thin and delicate and do not actively contract, and are therefore only mildly affected by physical and pharmacological stimuli. The most common cause of pulmonary arterial hemorrhage is ulceration of the vessel wall caused by destruction of the pulmonary parenchyma (lung cancer, necrotizing bacterial pneumonia, mycetoma). In these cases, bleeding is often contained when a clot temporarily seals the lesion, but if the clot dissolves or the rupture progresses, relapse is more severe.5 Unfortunately, it is not always possible to determine in which vascular network the hemorrhage began.
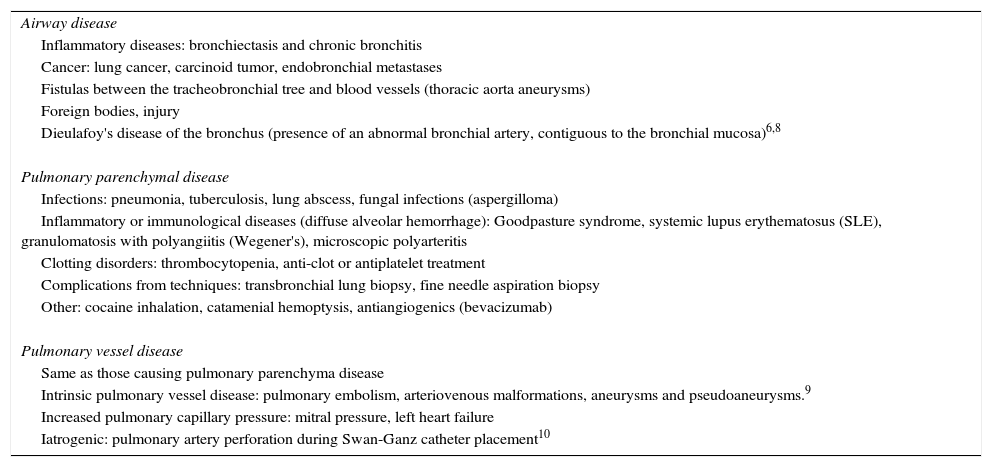
Etiology of HemoptysisThe underlying disease causing hemoptysis may involve the airway, the pulmonary parenchyma or the pulmonary veins themselves. The most common overall cause of hemoptysis is airway disease. The diseases which most commonly produce hemoptysis are bronchiectasis, chronic bronchitis and lung cancer, although this will vary depending of the population studied6,7 (Table 1).
Etiology of Hemoptysis.
| Airway disease |
| Inflammatory diseases: bronchiectasis and chronic bronchitis |
| Cancer: lung cancer, carcinoid tumor, endobronchial metastases |
| Fistulas between the tracheobronchial tree and blood vessels (thoracic aorta aneurysms) |
| Foreign bodies, injury |
| Dieulafoy's disease of the bronchus (presence of an abnormal bronchial artery, contiguous to the bronchial mucosa)6,8 |
| Pulmonary parenchymal disease |
| Infections: pneumonia, tuberculosis, lung abscess, fungal infections (aspergilloma) |
| Inflammatory or immunological diseases (diffuse alveolar hemorrhage): Goodpasture syndrome, systemic lupus erythematosus (SLE), granulomatosis with polyangiitis (Wegener's), microscopic polyarteritis |
| Clotting disorders: thrombocytopenia, anti-clot or antiplatelet treatment |
| Complications from techniques: transbronchial lung biopsy, fine needle aspiration biopsy |
| Other: cocaine inhalation, catamenial hemoptysis, antiangiogenics (bevacizumab) |
| Pulmonary vessel disease |
| Same as those causing pulmonary parenchyma disease |
| Intrinsic pulmonary vessel disease: pulmonary embolism, arteriovenous malformations, aneurysms and pseudoaneurysms.9 |
| Increased pulmonary capillary pressure: mitral pressure, left heart failure |
| Iatrogenic: pulmonary artery perforation during Swan-Ganz catheter placement10 |
In around 20% of cases of hemoptysis11,12 and up to 42% of smokers, an etiological diagnosis cannot be established after bronchoscopy and chest computed tomography (CT),6,7,11 and the case is classified as idiopathic or cryptogenic hemoptysis. Most of these patients are smokers and hemoptysis is generally caused by inflammation of the bronchial wall due tobacco smoke, rather than an undefined problem. Idiopathic hemoptysis is also related with chronic or acute bronchial inflammation, occult bronchiectasis, inactive tuberculosis, pulmonary vascular malformations, and coagulation disorders.12 The systematic use of CT can be expected to reduce the prevalence of hemoptysis of unknown cause.
DiagnosisInitial EvaluationSuspected hemoptysis in a patient must be confirmed, its severity established, the origin of bleeding located and the cause determined.
- 1.
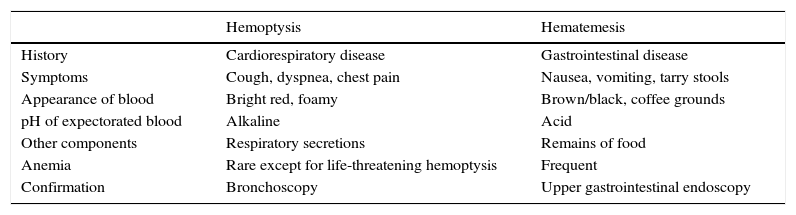
Confirmation of hemoptysis: this is based on the direct observation of bleeding or bleeding reported by the patient. Upper airway bleeding must be differentiated from hematemesis on the basis of accompanying symptoms, the appearance of the blood, and the patient's concomitant diseases (Table 2). The oral cavity and nasal fossa must be examined to ensure that the bleeding is subglottic, bearing in mind that nose or gum bleeds occurring during the night may be mistakenly taken for hemoptysis the next morning. Although the clinical data are usually sufficient, endoscopic examinations such as rhinolaryngoscopy, gastroscopy and/or bronchoscopy may be needed to confirm the origin of the bleeding.
Table 2.Differential Diagnosis Between Hemoptysis and Hematemesis.
Hemoptysis Hematemesis History Cardiorespiratory disease Gastrointestinal disease Symptoms Cough, dyspnea, chest pain Nausea, vomiting, tarry stools Appearance of blood Bright red, foamy Brown/black, coffee grounds pH of expectorated blood Alkaline Acid Other components Respiratory secretions Remains of food Anemia Rare except for life-threatening hemoptysis Frequent Confirmation Bronchoscopy Upper gastrointestinal endoscopy - 2.
Evaluation of severity: bleeding must be quantified. Severity can vary widely, from blood-streaked sputum, through gross hemoptysis, to life-threatening hemoptysis.3,13
- 3.
Location of origin and etiology: the origin of hemoptysis can be identified during the initial efforts to control bleeding or later, during diagnostic evaluation, once the patient has been stabilized. It is important to identify the cause of hemoptysis, even if hemorrhage has been stopped, because this will determine the appropriate definitive treatment.
Targeted clinical history and physical examination (Table 3) can provide the data needed to make an initial etiological orientation, evaluate severity of the hemoptysis and guide diagnostic and therapeutic measures, as required.
Physical Examination and General Laboratory Tests in the Patient With Hemoptysis.
| History | |
| Injury | Diagnostic manipulation in the airway/lung, chest injury, foreign body aspiration |
| Immobilization | |
| Toxic contact | Tobacco, asbestos, organic chemicals |
| Drugs | NSAIDs, antiplatelets, anti-clotting agents |
| Epidemiological | Trips, tuberculosis contact/risk, parasites |
| Family members | Clotting disorders, VTED, hemoptysis, brain aneurysms, epistaxis or gastrointestinal hemorrhage (THF) |
| Concomitant diseases | |
| Respiratory diseases | Criteria for chronic bronchitis, cough, expectoration, bronchorrhea, pneumonia, previous hemoptysis, cancer |
| Other diseases | Heart disease, VTED, immunosuppression, renal hematological, autoimmune or systemic disease |
| Laboratory tests | |
| Complete blood count | Anemia, leukocytosis |
| Clotting | Thrombocytopenia, clotting disorders, D-dimer |
| Arterial blood gases | Hypoxemia, hypercapnia |
| Biochemistry | Liver function, kidney function, tumor markers, proBNP, ANA, ENA, ANCA, anti-glomerular basement membrane antibodies |
| Urine | Sediment, proteinuria |
| Sputum | Cytology, microbiology: Gram, Ziehl-Neelsen, usual cultures and Lowëstein-Jensen |
ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; COPD, chronic obstructive pulmonary disease; ENA, extractable nuclear antigen antibodies; HHT, hereditary hemorrhagic telangiectasia; NSAID, non-steroidal anti-inflammatory drugs; proBNP, brain natriuretic peptide; VTED, venous thromboembolic disease.
Initial complementary tests must include (Table 3):
- 1.
Clinical laboratory tests with complete blood count, coagulation parameters and biochemistry.
- 2.
Pulse oximetry and arterial blood gases to determine the impact of hemoptysis on oxygenation and ventilation.
- 3.
Spirometry: active hemoptysis is an absolute contraindication to spirometry testing.14 Once bleeding is controlled, spirometry is used to determine the patient's respiratory function, which is essential if the patient is a candidate for deferred surgical intervention (strong recommendation, 1C).
- 4.
Electrocardiogram: particularly if heart disease or pulmonary thromboembolism are suspected.
- 5.
Transthoracic echocardiogram: to detect endocarditis, mitral valve stenosis, congenital heart diseases, signs of pulmonary hypertension, or the presence of shunts due to arteriovenous malformations.
- 6.
Cytological study and sputum microbiology.
- 7.
Mantoux in patients with suspected tuberculosis, and blood cultures or serologies if infectious disease is suspected.
Anterior-posterior and lateral chest X-rays are the initial imaging tests performed in patients with hemoptysis. The information they provide, however, is scant,15 and results can be normal in patients with bronchiectasis and malignant disease, 2 of the most common causes of bleeding.4,6
Chest multidetector CT (MDCT) must be performed in all patients with gross hemoptysis, in those with blood-streaked sputum and suspected bronchiectasis or risk factors for lung cancer (>40 years of age with an cumulative tobacco consumption of >30 pack-years), and those with pathological findings on X-ray. The type of CT used is different in each of the following clinical situations:
- -
Patients with blood-streaked sputum and suspected bronchiectasis: volumetric high resolution CT (HRCT) of the chest, without intravenous contrast medium.
- -
Patients with blood-streaked sputum and high risk factors (smoker, COPD) or with pathological findings on chest X-ray: MDCT with intravenous contrast medium6,15 (strong recommendation, 1A).
- -
Patients with life-threatening hemoptysis and active bleeding, candidates for embolization: angio-MDCT, from the base of the neck to the renal arteries4 (weak recommendation, 2C).
Angio-MDCT is a non-invasive technique which accurately identifies the presence, origin, number and trajectory of the thoracic, bronchial and non-bronchial arteries and pulmonary arteries which may be the source of bleeding. It locates the site of the bleed in up to 70%–88% of cases.16,17 This technique generates a very accurate vascular map which is useful during the angiography embolization procedure,6,18 and also evaluates the cause of bleeding and its impact on the pulmonary parenchyma and the airway.4,18
The systemic and pulmonary arteries are identified using thin-slice axial reconstructions, multiplanar reconstructions, maximum intensity projections, and volume-rendered images.19,20 High resolution reconstructions of the pulmonary parenchyma are useful for evaluating the cause of bleeding and its impact on the parenchyma, and multiplanar and volumetric reconstructions are useful for evaluating the airway.9,21
Bronchial arteries are considered pathological if they are ≥2mm in diameter, tortuous, and if their trajectory can be identified from their origin to the pulmonary hilum. Angio-MDCT detects 100% of the pathological bronchial arteries observed on standard angiography and provides a more accurate view of ectopic bronchial arteries.9,18–21
Non-bronchial systemic arteries are considered pathological if they are ≥2mm in diameter and tortuous; visualization in extrapleural fat, associated with pleural thickening ≥3mm and changes in underlying pulmonary parenchyma, indicates that they are the cause of bleeding in patients with life-threatening hemoptysis. Angio-MDCT detects 62%–97% of the non-bronchial arteries seen on standard angiography.4,18–21
Pulmonary arteries are a source of bleeding in at least 10% of cases, and half of these patients have hypertrophic bronchial arteries. In chronic pulmonary thromboembolism, hemoptysis does not originate in the pulmonary arteries, but instead is due to bronchial hypervascularization secondary to pulmonary ischemia.22
The cause of hemoptysis (tumor, bronchiectasis, aspergilloma) can be identified in high resolution axial reconstructions; these images can also reveal the areas of bleeding in the form of ground glass opacities and consolidations and clots in the bronchial lumens, with or without distal atelectasis.18
Angio-MDCT has replaced angiogram as the diagnostic method for identifying arteries which are the source of bleeding in hemoptysis.22 Angiogram is now performed only as a procedure previous to the embolization of pathological arteries detected on angio-MDCT21 and if bleeding persists after bronchoscopy; however, in hemodynamically unstable patients, angiogram can be performed directly without prior angio-MDCT.
Imaging techniques have some limitations, for example, in unstable patients, patients with active bleeding who require endobronchial treatment and patients with bilateral radiological abnormalities in whom radiological localization of the bleeding is a challenge.
Diagnostic BronchoscopyFlexible bronchoscopy has a fundamental role in the diagnosis of hemoptysis, from blood-streaked sputum to gross hemoptysis. It can be performed rapidly at the patient's bedside (in the ICU), and not only is it beneficial in immediately controlling bleeding, but also its yield in locating the source of bleeding is high.
Bronchoscopy has several objectives:
- 1.
Confirmation of hemoptysis.
- 2.
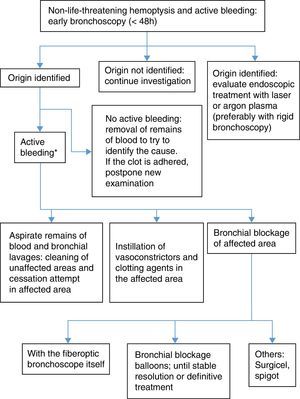
Localization of bleeding. Flexible bronchoscopy locates the origin of bleeding in 73%–93% of cases.17,23 Studies have shown that the source of active bleeding is more likely to be located when bronchoscopy is performed during active hemoptysis or within 24–48h after cessation, rather than when bronchoscopy is delayed, although the diagnostic yield is similar in both cases23–25 (strong recommendation, 1C). In the case of life-threatening hemoptysis, a bronchoscopy must be performed as soon as possible if the patient is unstable and after intubation,13,26 since, in addition to monitoring the airway, the bronchoscope can be withdrawn if oxygenation deteriorates or the bronchoscope is obstructed by clots. Rigid bronchoscopy is not generally used for the diagnosis and initial evaluation of life-threatening hemoptysis, since it needs to be performed in the operating room under general anesthesia, and, unlike flexible bronchoscopy, cannot be used for visualizing the distal airway. The active bleed must be visualized in order to locate the origin of the hemoptysis. This will accurately identify the bronchus or the bronchial area involved. Bloody remains and clots are often encountered. Locating clots does not firmly guarantee that the origin of the bleed has been pinpointed, but a greater concentration and adherence of clots in a certain bronchus, corroborated by imaging techniques, may indicate the involved area. Bloody remains must be aspirated with repeated small bronchial lavages, in order to improve patency and allow the underlying territory to be examined. However, if fresh adhered clots are seen, no great efforts should be made to immediately remove them, in view of the risk of new bleeding. The examination may be repeated later, when they have progressed and can be removed with less risk.
- 3.
Diagnosis of cause of bleeding. Bronchoscopy can be used to perform an endobronchial inspection and to determine the appearance of the mucosa: capillary vascular hypertrophy or malformation, areas of inflammatory or infiltrative thickening of the mucosa, bronchial stenosis, endobronchial tumors, anthracosis or anthracostenosis, bronchiolitis, etc. Changes are often non-specific, and as such, non-diagnostic.27
In addition to the visual examination, flexible bronchoscopy can be used to collect samples for cytology and microbiological studies, bronchial aspirate, bronchoalveolar lavage if alveolar bleeding is suspected, and biopsies and/or bronchial brushing, if malignancy is suspected. If lesions are highly vascularized, some authors recommend the instillation of 1–2ml of adrenaline diluted at 1:20000 before samples are collected, to reduce the risk of new bleeding, although the clinical evidence is low28 (weak recommendation, 2C).
Treatment of HemoptysisGeneral MeasuresThe severity of the patient must be determined in the initial evaluation, and a decision should be made regarding where he/she should be treated.
Patients with blood-streaked sputum generally do not need to be hospitalized, unless required by the cause of the hemoptysis. The etiological study may be performed gradually, hand in hand with the chosen treatment strategy.
Patients with gross hemoptysis generally need to be hospitalized. Outpatient treatment may be considered in patients in good general condition and a suspected cause that can be followed up in outpatient clinics, provided bleeding has stopped. Along with the general and symptomatic measures, etiological treatment (antibiotics, if infection is suspected), rest at home and outpatient monitoring after 24–48h are recommended. If the origin of bleeding is not clarified, clinical follow-up is recommended.
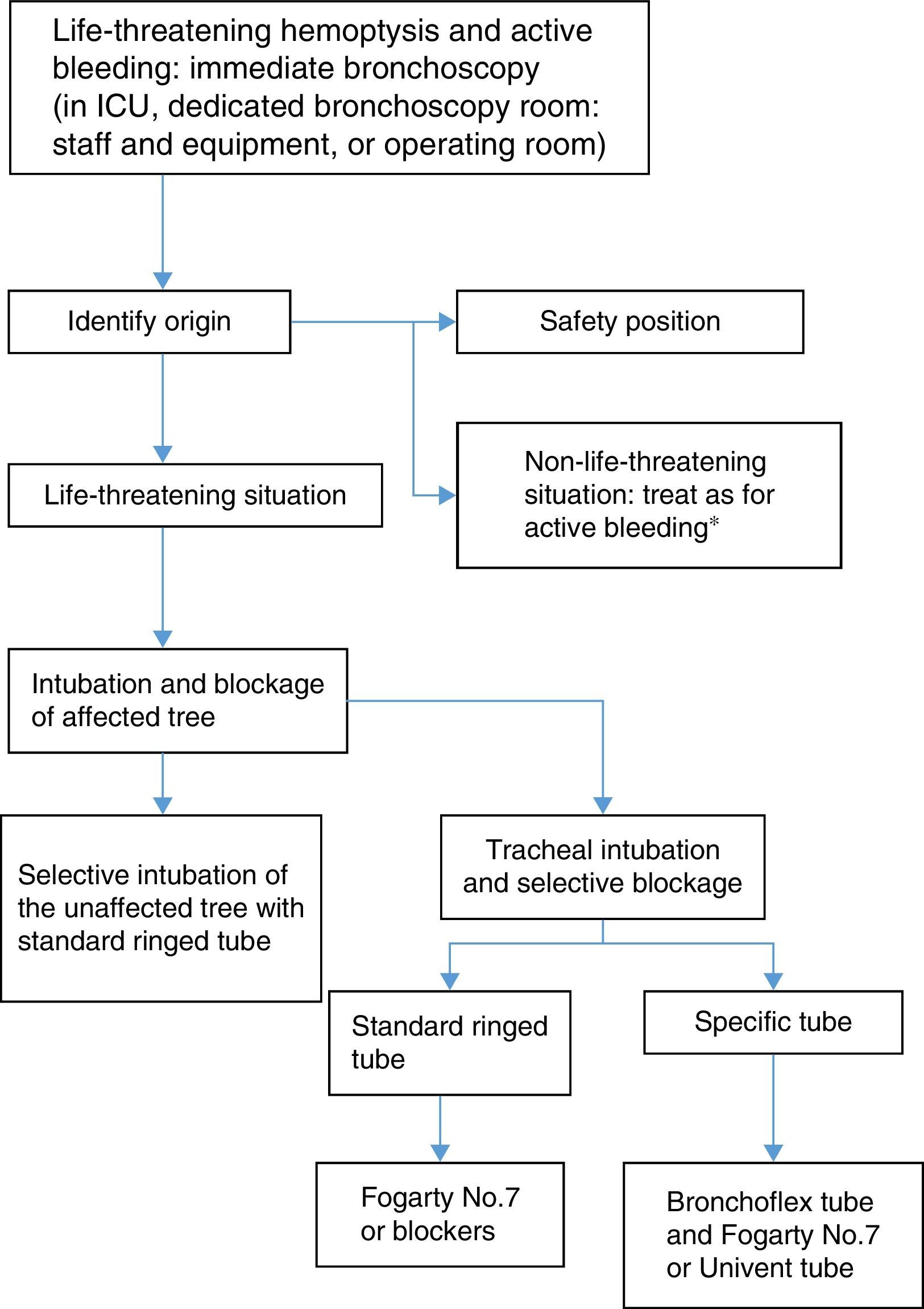
Life-threatening hemoptysis must be treated in the hospital, under close observation in intensive care units or pulmonology wards. A rapid and accurate simultaneous diagnosis of the cause and site of the bleeding is essential to facilitate control. Treatment is directed at ensuring airway patency and oxygenation, locating and stopping bleeding, achieving hemodynamic stability and identifying and treating the cause of the hemoptysis.1,29–31
Hospital management of active hemoptysis includes a series of general measures:
- 1.
Lateral decubitus bed rest on the affected side, in order to protect the airway and avoid aspiration of blood into the unaffected lung. Therefore, the hemithorax in which the bleeding is originating must first be determined. Clinical signs and symptoms and chest X-ray findings can be used to narrow down the site of the lesion.
- 2.
Monitoring of vital signs (blood pressure, heart and breathing rate, oxygen saturation) and quantification of the hemoptysis.
- 3.
Supplementary oxygen, if required.
- 4.
Administration of antitussives to control coughing. Chest physical therapy techniques must be avoided.
- 5.
Empiric antibiotic treatment, useful in hemoptysis associated with respiratory infection and, in general, to prevent subsequent complications.
- 6.
Total fasting to avoid bronchoaspiration and to facilitate the performance of urgent tests, such as bronchoscopy, CT or angiogram.
- 7.
Availability of a blood reserve and placement of large caliber venous access for fluid management, and if required, red blood cell transfusion.
- 8.
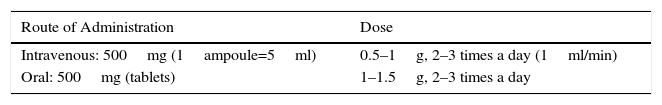
Antifibrinolytics (aminocaproic acid, tranexamic acid [TA]): these act by inhibiting the process of clot dissolution, which in turn reduces bleeding. A Cochrane review32 identified 2 clinical trials which evaluate the use of oral and intravenous TA (Anchafibrin®). There is insufficient data to recommend its use, but some small studies suggest that it may reduce hemorrhage duration. However, a recent best evidence topic review33 concludes that, while a recommendation with strong evidence cannot be given, TA can reduce both the duration and volume of bleeding, with a low short-term risk of thromboembolic disease (weak recommendation, 2B) (Table 4).
- 9.
Aminocaproic acid (Capramin®) has been used in isolated case series, particularly in the intracavitary treatment of aspergillomas.34,35 No randomized controlled studies have been performed to determine its efficacy.
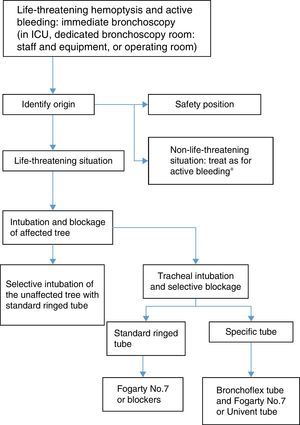
If the patient cannot eliminate blood from the tracheobronchial tree or if respiratory failure is severe, measures must be taken to protect the airway. Orotracheal intubation with a large diameter tube (8–9mm) facilitates diagnostic and interventional bronchoscopy. For control of bleeding, blockage of the bleeding bronchial segment may also be necessary to preserve the ventilation of the healthy lung.25,36 Blockage can be performed with the endotracheal tube itself. In the case of bleeding in the right bronchial tree, the left main bronchus can be selectively intubated using the bronchoscope, so that that the inflatable cuff of the endotracheal tube completely isolates the left lung.
There are other systems for blocking the bronchi:
- 1.
Bronchial blockers which prolong the tube itself, such as the Torque Control Blocker Univent®.
- 2.
Independent bronchial blockers which are placed using a standard tube:
- a)
Fogarty-type catheter with inflatable balloon (No. 7 or larger).
- b)
Arndt Endobronchial Blocker Catheter®, temporarily attached to the tip of the bronchoscope to guide it to its location (No. 7–9).
- c)
EZ-Blocker®, with a Y-shaped distal tip to facilitate anchoring in the tracheal carina, and 2 balloons which can be inflated independently.
- d)
Cohen Flexitip Endobronchial Blocker®, with a balloon cuff and flexible tip to aid placement.
- a)
- 3.
Broncoflex® tube, with a working channel through which a Fogarty catheter or similar can be introduced, and an external fixation system. Its advantage is that the internal caliber of the tube is kept clear, so that the balloon can be easily placed in any of the main bronchi.
- 4.
Single-lung blockage via selective intubation using a double-lumen tube. This system is less recommendable, because it cannot be used to introduce standard fiberoptic bronchoscopes, and it is more difficult to place due to poor visibility in the site of active bleeding.
Therapeutic bronchoscopy is indicated to respond to a risk situation, generally in the context of a life-threatening hemoptysis.
Flexible bronchoscopy is the initial procedure of choice in patients with life-threatening hemoptysis and hemodynamic instability in which control of bleeding is essential. Flexible bronchoscopy has an advantage over CT or rigid bronchoscopy in that it can be performed wherever the patient needs to be treated.
However, rigid bronchoscopy in combination with flexible bronchoscopy is the most comprehensive and safest procedure in life-threatening hemoptysis36,37 (strong recommendation, 1C), since it can be used to:
- 1.
Ensure the patient is adequately ventilated.
- 2.
Ensure patency of the airway by aspiration of bloody remains with large-caliber tubes.
- 3.
Perform hemostasis directly on the areas of bleeding by applying pressure with the external wall of the distal tip of the rigid bronchoscope, or with the administration of vasoconstrictors or endobronchial clotting agents.
- 4.
Access the distal bronchial tree with use of the flexible bronchoscope.
Despite these advantages, flexible bronchoscopy is still the most widely used tool in these cases, because access to rigid bronchoscopy is more limited: fewer bronchoscopists are trained in its use, and it should be performed in the operating room under general anesthesia.
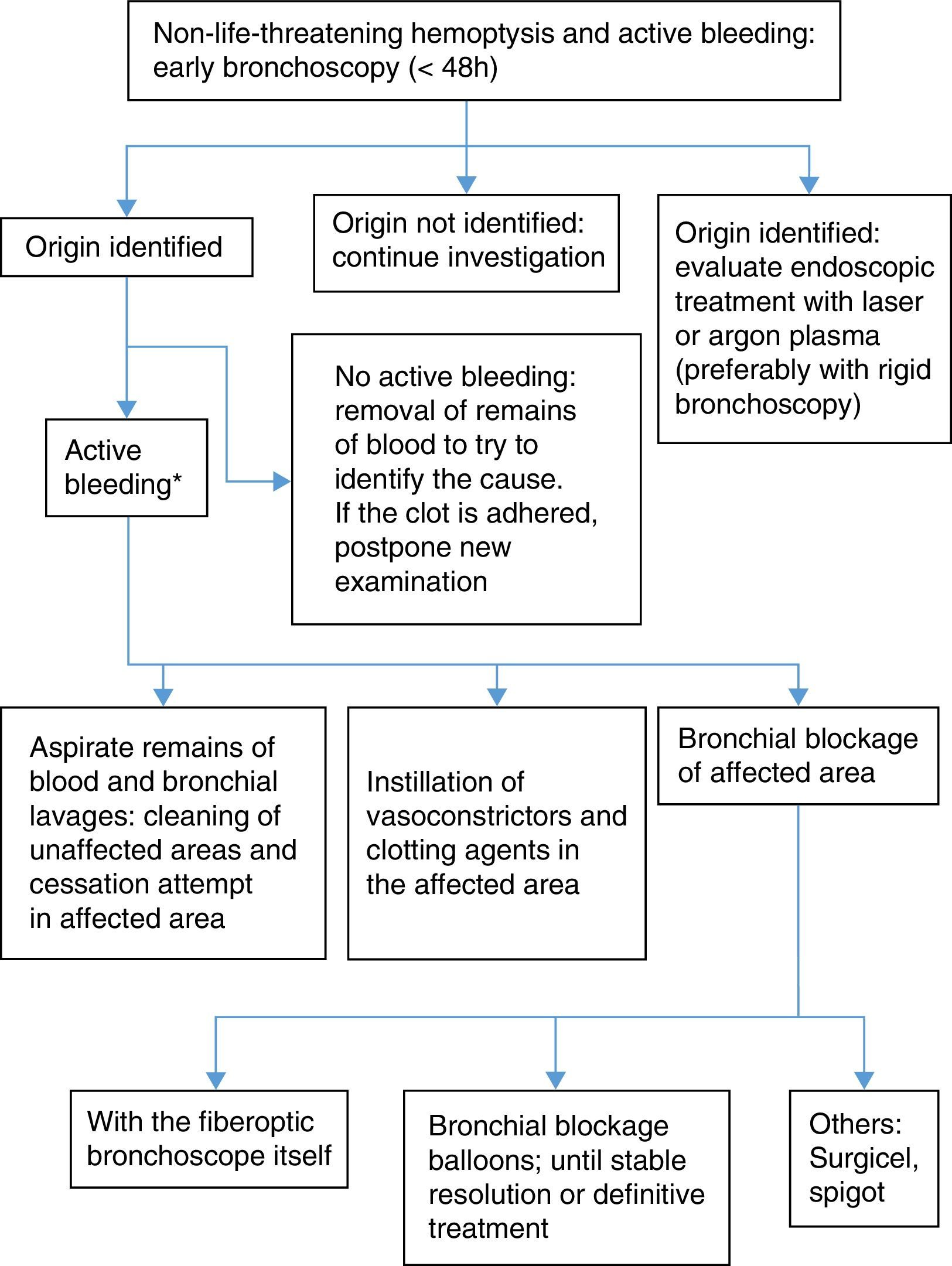
When the origin of the hemoptysis has been identified, and the bleeding is not severe enough to warrant blockage of the entire bronchial tree, local measures can be applied. Evidence of their efficacy is not conclusive and they have not been evaluated in clinical trials:
- 1.
Bronchial blockage with flexible bronchoscope and continued aspiration to collapse the segment and inhibit hemorrhage.
- 2.
Selective bronchial blockage by the introduction of a balloon catheter via the working channel.
- a)
Fogarty-type balloon catheter No. 5 (5Fr.)38 or similar (occlusion balloon catheters Olympus B5-2C® and B7-2C®).
- b)
Longer catheters such as the Olympus Multi-3V Plus B-V232P-A® balloon catheter, which allow extraction of the bronchoscope without dislodging the balloon.
- a)
- 3.
Selective bronchoscope-guided bronchial blockage without occupying the working channel. A guide is introduced via the working channel until it reaches the selected bronchus, and after withdrawing the bronchoscope, a balloon catheter is placed using the guide. Although this procedure is more complicated, the balloon catheter can be left in place, while the bronchoscope is withdrawn.39
The balloon must be kept inflated for 24–48h for the clot to form, although it can be left in the airway for up to several days. To prevent ischemia of the mucosa, it must be regularly deflated, at least 3 times a day,38 always under endoscopic visualization, so that it can be reinflated immediately if bleeding persists. If bleeding does not start again after several hours, the balloon catheter can be withdrawn.
- 4.
Bronchial lavage with cold saline solution (4°C) using 50ml aliquots until bleeding ceases, without exceeding a total volume of 500ml.40 The mechanism of action is local vasoconstriction, although no controlled studies are available to confirm efficacy24 (weak recommendation, 2C).
- 5.
Instillation of hemostatic agents:
- a)
Vasoconstrictors: adrenaline diluted at 1:20000 administered via the working channel in increments of 1ml. This has not been compared in controlled trials and is only supported by clinical experience. To minimize cardiovascular effects in at-risk patients, some authors have suggested replacing adrenaline with antidiuretic hormone, such as terlipressin or ornipressin, although evidence is anecdotal41,42 (weak recommendation, 2C).
- b)
Undiluted tranexamic acid applied on the focus of bleeding at an initial dose of 500mg43,44 (weak recommendation, 2C).
- c)
Fibrinogen-thrombin (Tissucol®). This has been used in 2 case series of hemoptysis in which previous endoscopic procedures had failed45 (weak recommendation, 2C).
- d)
Topical hemostatics are not useful in very copious hemoptysis, since the blood acts as a form of “lavage” on these products, diminishing their efficacy.
- a)
- 6.
Other bronchial blockage systems: oxidized regenerated cellulose mesh (Surgicel®) or silicone plugs (Watanabe spigot). These have been used with success in case series.46,47
- 7.
In cases of bleeding tumors, accessible and visible by endoscopy, various clotting therapies can be used:
- a)
Laser photocoagulation (Nd:YAG, Nd:YAP, diode laser): the efficacy of these techniques in stopping hemorrhage ranges from 60% to 74%, although bleeding is reduced in up to 94% of cases.48,49 Results are not so favorable if bleeding is abundant50 (strong recommendation, 1C).
- b)
Argon plasma electrocoagulation. Argon plasma immediately stopped hemorrhage in 100% of actively bleeding endobronchial lesions in a patient series.51 It is useful if cough can be avoided and bleeding is not very abundant (weak recommendation, 1C) (Figs. 1 and 2).
- a)
The safest and most effective management strategy in most cases of massive or recurrent hemoptysis is endovascular embolization; it is generally definitive, or else is useful for stabilizing the patient before surgery. Embolization is indicated in all patients with life-threatening or recurrent hemoptysis in whom pathological arteries are observed on angio-MDCT; it must be performed by expert interventional radiologists with extensive knowledge of the potential drawbacks of the procedure, in a dedicated interventional vascular radiology room with digital subtraction equipment21 (strong recommendation, 1B).
The most frequently embolized arteries are the bronchial arteries. Angiographic criteria to determine that the systemic arteries, whether bronchial or non-bronchial, are the source of bleeding and require embolization are: tortuous, dilated arteries, peribronchial hypervascularity or neovascularity, shunts from systemic arteries to pulmonary arteries or veins, extravasation of contrast medium and bronchial artery aneurysm.4,18–21
The catheter is guided selectively to the pathological artery via the femoral artery, using the Seldinger technique. The materials used for the occlusion of small distal arteries vary widely, but the most typical are calibrated non-resorbable polyvinyl alcohol particles or gelatin-coated acrylic polymer microspheres.52 Coils and other metal devices are reserved for the treatment of pulmonary artery aneurysms and arteriovenous malformations; they are not generally used as a single treatment in the embolization of bronchial arteries because they cause proximal arterial occlusion and can obstruct re-embolization for recurrent hemoptypsis.21
The most serious complication of bronchial artery embolization is medullary ischemia due to inadvertent occlusion of a spinal artery, particularly the artery of Adamkiewicz, when it arises from the thoracic artery between levels T5 and T8.53 The risk of this complication is reduced when embolization of the bronchial artery is performed superselectively with microcatheter.54
For good hemoptysis management, all pathological arteries must be embolized (strong recommendation, 1B). Hemoptysis recurs in 10%–55% of patients, due to incomplete embolization, failure to embolize all bleeding arteries, recanalization of the embolized vessel or revascularization due to collateral circulation caused by progression of the underlying disease21,52 Most failures involve cases of mycetomas.55
Surgical TreatmentHistorically, pulmonary surgical resection was the only option available for the treatment of localized life-threatening hemoptysis which did not respond to medical treatment. However, the mortality rate of surgery performed under emergency conditions is high, ranging between 25% and 50%.55,56 The difficult conditions under which this surgery is performed during active, massive hemorrhage, and the frequent need to perform pneumonectomy, are factors known to increase the risk of postoperative complications (principally bronchopleural fistula and empyema) and death.
Currently, surgery is reserved for life-threatening hemoptysis in situations in which the cause of bleeding can be treated by the intervention and the origin of the bleeding has been specifically and reliably located (strong recommendation, 1B).57
In most cases, hemoptysis originates in the bronchial arteries. In this case, emergency surgery can be performed, if the resection is carried out during active, uncontrollable bleeding, or else it can be programmed to be performed during the same hospital stay, if bleeding has previously been controlled, or planned (within 6 months following discharge), in patients with suspected high risk of recurrent hemoptysis.56 Complications and mortality rates are much higher when surgical resection is performed during active hemorrhage, and significantly reduced in patients in whom surgery can be delayed following cessation of bleeding using arterial embolization and support measures5,56,58,59 (strong recommendation, 1B).
The absence of tracheobronchial hemorrhage increases the safety of the surgical intervention. In this case, the hemoptysis is more confined and a more economical resection can be performed. Cleaning the bronchial tree with flexible or rigid bronchoscopy contributes to parenchymal recovery and pulmonary vascularization, making it easier to perform the surgery.
Although lung resection is not proposed as an initial treatment of hemoptysis of vascular origin, there are situations in which emergency surgery is the only possible alternative; for example, when effective embolization is impossible, bleeding recurs within 72h of completing the procedure, or in patients with a recent history of previous embolization of life-threatening hemoptysis.
Surgical interventions must also be undertaken urgently in cases in which hemorrhage of the pulmonary arteries is suspected,5 generally due to ulceration of the vessel wall caused by a destructive pulmonary process (lung cancer, necrotizing pneumonia, pulmonary mycetoma).
Only 1 multicenter study has examined the results of surgical resection in hemoptysis classified as urgent.60 Even with the limitations inherent in the collection of data in a national registry – the Nationwide Inpatient Sample – it is interesting to note that, of a series of almost half a million admissions for hemoptysis, less than 1% underwent surgery. Overall mortality in this group was 8.1%, lower than reported in the literature, even the most modern series,56,59,60 but significantly higher than in patients undergoing programmed lobectomy or pneumonectomy for any other cause.
Special SituationsHemoptysis With Normal Chest RadiographA normal chest radiograph obtained in the context of hemoptysis does not exclude the possibility of malignancy or other underlying disease.4,12,16,61 Incidence of malignancy in patients with hemoptysis and normal chest radiograph is generally low, but can be as high as 10% in patients aged over 40 years with a history of smoking,62 even if hemoptysis is mild,63 indicating the need for further examinations, as recommended by the National Lung Screening Trial (NLST)64 (strong recommendation, 1A). Bronchoscopy can detect an endobronchial lesion in 5% of patients with mild hemoptysis and normal chest radiograph,65 and HRCT detects bronchiectasis in up to 70% of cases of severe hemoptysis and normal chest radiograph.17 Depending on the type of hemoptysis, then, various additional tests may be required.
- 1.
Blood-streaked sputum: in the absence of risk factors for cancer, additional testing will only be performed if hemoptysis recurs or the volume of blood increases.65 In patients with recurrent hemoptysis, the first step is to perform a chest CT (HRCT or MDCT). Performing CT before bronchoscopy is justified for several reasons: bronchiectasis and small tumors may be missed on bronchoscopy, while CT might be diagnostic of bronchiectasis and arteriovenous malformations which might rule out the need for a bronchoscopy; and finally, CT might be useful for selecting the most beneficial diagnostic endoscopic technique (flexible bronchoscopy or ultrasound-guided bronchoscopy)6,17,66,67 (strong recommendation, 1B).
- 2.
Gross hemoptysis: if the cause is unknown, a bronchoscopy is required, particularly in patients with risk factor for malignancy. However, depending on the stability of the patient, a preliminary chest CT may be recommended. Both CT and bronchoscopy have advantages, depending on the clinical situation, so the 2 procedures are complementary.16,66 CT is better for diagnosis of bronchiectasis or cancer, while bronchoscopy is better for detecting small abnormalities in the mucosa, such as acute bronchitis or Kaposi's sarcoma67,68 (strong recommendation, 1B). Bronchoscopy has the additional advantage of providing specimens for analysis in the pathology laboratory.67 The combined use of bronchoscopy and MDCT increases the diagnostic yield for the origin of the bleeding.17
If CT is normal, bronchoscopy may help diagnose the cause of bleeding in up to 16% of cases, or in as many as 37% if the patient's clinical history is also taken into account.62 If the bronchoscopy is negative, the patient is classified as having cryptogenic hemoptysis. A combination of negative CT and bronchoscopy has a very low chance of being malignant (1%) after a follow-up of 6 months.69 A follow-up MDCT is only advisable several weeks or months after an acute episode of hemoptysis to evaluate the progress of parenchymal changes which may hide intracavitary lesions such as mycetomas or to detect small malignant lesions initially masked by the endobronchial or parenchymal bleeding.4,63,69
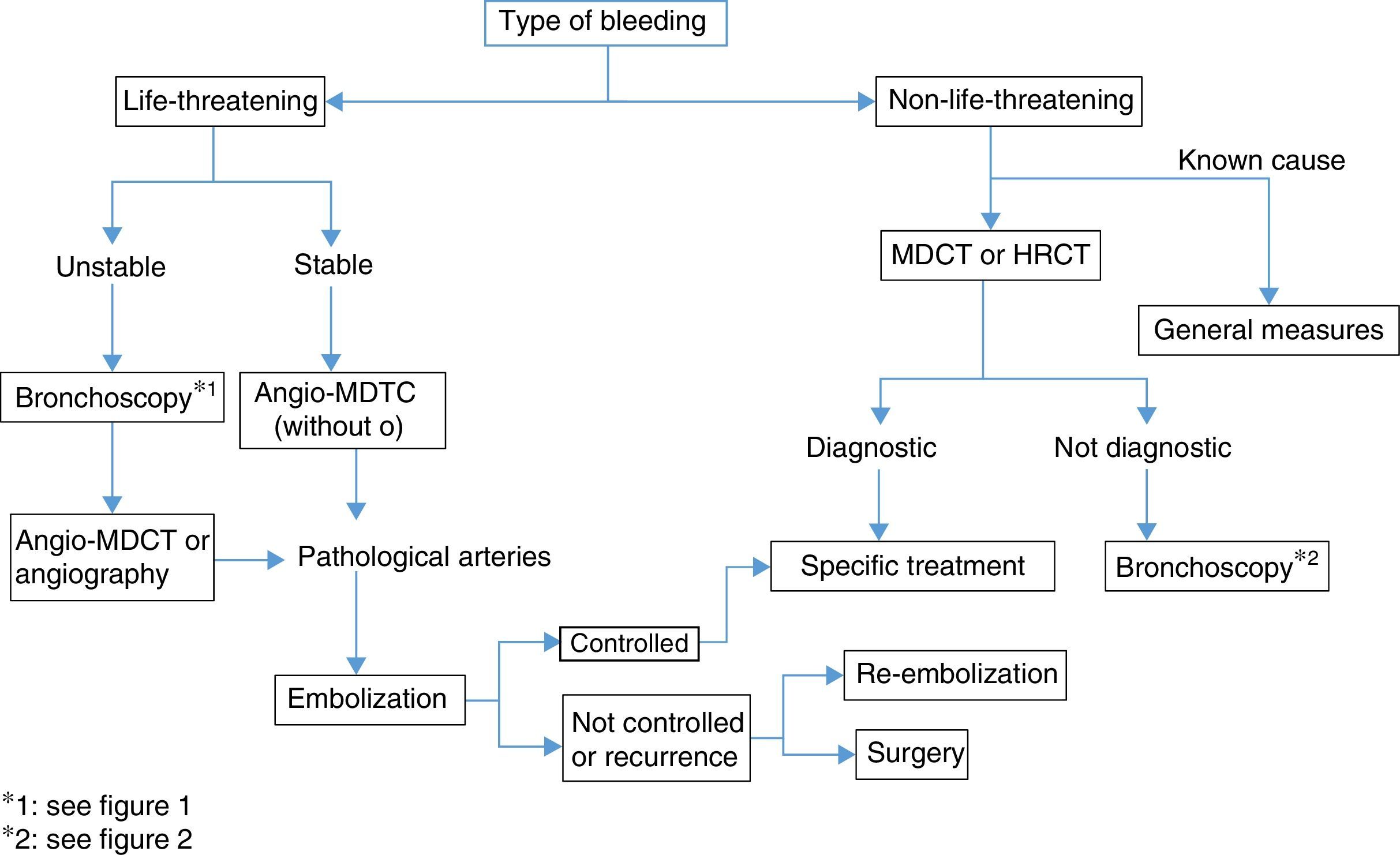
Life-Threatening HemoptysisThe management strategy and actions to be taken in the case of life-threatening hemoptysis have been discussed above in various sections of these guidelines. To summarize, the importance of identifying the cause must be underlined. This is generally determined while the hemorrhage is being brought under control, or even when bleeding has stopped, since the cause of the event will guide the choice of definitive treatment.
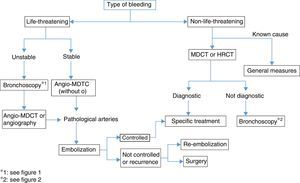
Angio-MDCT is the first diagnostic test to be undertaken in patients with life-threatening hemoptysis and active bleeding, except for patients in whom bleeding must be controlled and the airway ensured, in which case bronchoscopy will be the first test (weak recommendation, 2C). If the bronchoscopy is not diagnostic, the next step is angio-MDCT, to identify the cause and also the arteries from which the bleeding originates; this should be performed prior to embolization. In hemodynamically unstable patients, who require urgent treatment, an angiogram, useful for both diagnosis and treatment (embolization), can be performed directly (Fig. 3).
Some procedures may have been performed to control bleeding, and may need to be repeated in a subsequent diagnostic evaluation, since the visualization of bleeding and diagnostic sensitivity during the acute phase are not optimal.4
Hemoptysis in Lung Cancer PatientsHemoptysis occurs in 7%–10% of patients with lung cancer, and is more common in central airway lesions than in peripheral lesions of the pulmonary parenchyma. Bleeding may be due to various mechanisms: neovascularization, exfoliation of the tumor surface with exposure of underlying vessels, tumor necrosis, injury caused by cough, iatrogenic procedures (bronchoscopy), and the formation of fistulas from the vessels to the airway.
Mild hemoptysis does not usually require any bronchoscopic procedure, but hemoptysis involving larger amounts of blood may need endoscopic treatments, embolization or even surgery.
Life-threatening hemoptysis in patients with lung cancer has a much poorer prognosis than when it is due to other causes. Mortality among these patients ranges from 59% to 100%. Surgery in these cases is not usually an alternative, since the patients generally present with advanced disease, so are not candidates for resection.
Bronchoscopy can be performed for diagnostic and therapeutic purposes. If no direct cause of bleeding is observed, as is usual with peripheral tumors, bronchoscopic management consists of engaging the bronchoscope and instilling cold saline solution into the bronchus of origin of the hemorrhage, in addition to the other methods discussed above. If these measures are not useful, bronchial artery embolization should be undertaken.70,71 If bleeding lesions are observed on bronchoscopy, they can be treated with YAG-laser or argon plasma. Contact electrocautery may also be effective, but it has only been used anecdotally and no data are available to recommend its use57 (strong recommendation, 1C).
When hemoptysis in cancer patients is not life-threatening and the tumor is operable, the best treatment is surgical resection, provided this is not contraindicated. If the tumor is inoperable, external radiation therapy can be applied in the case of a endobronchial or peripheral tumor57,72 (strong recommendation, 1C). Endobronchial brachytherapy can be useful for endoluminal lesions, if there is no ulceration of the tumor mucosa, since this is a contraindication for this technique.73 However, there are insufficient studies supporting its use in controlling bleeding in lung cancer patients. Most studies explore the palliative treatment of symptoms, including hemoptysis, but the heterogeneity of the patients makes it difficult to draw conclusions.74
Please cite this article as: Cordovilla R, Bollo de Miguel E, Nuñez Ares A, Cosano Povedano FJ, Herráez Ortega I, Jiménez Merchán R. Diagnóstico y tratamiento de la hemoptisi. Arch Bronconeumol. 2016;52:368–377.