Diabetes mellitus (DM), a very common disease in Mexico, is a well-known risk factor for tuberculosis (TB). However, it is not known by which extent DM predisposes to adverse events (AE) to anti-TB drugs and/or to worse outcomes in patients with multidrug-resistant (MDR-TB) and extensively drug-resistant TB (XDR-TB). The main objective of this study was to describe the outcomes of TB treatment, the impact of DM and the prevalence of AE in a cohort of patients with MDR-/XDR pulmonary TB treated at the national TB referral centre in Mexico City.
ResultsNinety patients were enrolled between 2010 and 2015: 73 with MDR-TB (81.1%), 11 with pre-XDR-TB (12.2%) and 6 (6.7%) with XDR-TB, including 49 (54.4%) with DM, and 3 with Human Immunodeficiency Virus (HIV) co-infection (3.3%). In 98% of patients, diagnosis was made by culture and drug susceptibility testing, while in a single case the diagnosis was made by a molecular test. The presence of DM was associated with an increased risk of serious drug-related AEs, such as nephrotoxicity (Odds Ratio [OR]=6.5; 95% Confidence Interval [95% CI]: 1.9–21.8) and hypothyroidism (OR=8.8; 95% CI: 1.8–54.2), but not for a worse outcome.
ConclusionsOur data suggest that DM does not impact second-line TB treatment outcomes, but patients with DM have a higher risk of developing serious AEs to drug-resistant TB treatment, such as nephrotoxicity and hypothyroidism.
La diabetes mellitus (DM), una enfermedad muy frecuente en México, es un factor de riesgo bien conocido para el desarrollo de tuberculosis (TB). Sin embargo, se desconoce en qué medida la DM predispone al desarrollo de reacciones adversas (RA) a los fármacos anti-tuberculosis y/o si predispone a un peor resultado en pacientes con pacientes con TB multirresistente (TB-MR) y TB extremadamente resistente (TB-XR). El objetivo principal de este estudio fue describir los resultados del tratamiento anti-tuberculosis, el impacto de la DM y la prevalencia de RA en una cohorte de pacientes con TB pulmonar MR/XR tratados en el centro de referencia nacional para TB, en la Ciudad de México.
ResultadosEntre 2010 y 2015 se incluyeron 90 pacientes —73 con TB-MR (81,1%), 11 con TB pre-XR (12,2%) y 6 (6,7%) con TB-XR—, 49 (54,4%) de los cuales tenían DM y 3 con co-infección por el virus de la inmunodeficiencia humana (VIH) (3,3%). El diagnóstico se realizó mediante cultivo y pruebas de fármaco-sensibilidad (PFS) en el 98% de los pacientes y mediante prueba molecular en un caso. La presencia de DM se asoció con un mayor riesgo de RA graves, tales como nefrotoxicidad (odds ratio [OR]=6,5; intervalo de confianza del 95% [IC 95%]: 1,9–21,8) e hipotiroidismo (OR=8,8; IC 95%: 1,8–54,2), aunque no con peor resultado del tratamiento.
ConclusionesNuestros datos sugieren que la DM no tiene un impacto sobre los resultados del tratamiento anti-tuberculosis de segunda línea, pero los pacientes con DM tienen mayor riesgo de presentar RA graves secundarias al tratamiento, tales como nefrotoxicidad e hipotiroidismo.
The Region of the Americas accounts for <10% of the global total of tuberculosis (TB) cases, the lowest burden of TB in the world1; however, it is among the regions with the highest prevalence of diabetes mellitus (DM): 11.4% according to the International Diabetes Federation.2 DM is a known risk factor for the development of TB (it increases the risk between 2 and 4 fold) depending on the population.3
During the last decade, a decreasing trend in TB cases has been reported in Mexico; however, there is also a persistent increase in cases of multidrug-resistant tuberculosis (MDR-TB; Mycobacterium tuberculosis strain resistant to, at least, isoniazid and rifampicin)4 and extensively drug-resistant TB(XDR-TB) (an MDR strain with additional resistance to a fluoroquinolone and to, at least, one second-line injectable drug).5
Mexico, in particular, is facing an overall increasing rate of DM, from 5.8% in 2000 to 9.2% in 2012.6 To date, it is not clear by which extent DM predisposes to worse outcomes in MDR-TB patients and/or to adverse events (AE) of anti-TB drugs.
The main objective of this study, therefore, was to describe the outcomes of TB treatment, the impact of DM and the prevalence of AE in a cohort of patients with MDR/XDR pulmonary TB treated at the national TB referral centre in Mexico City.
MethodsThe study was performed under a cooperative project, which involved the Mexican National Tuberculosis Programme, the Instituto Nacional de Enfermedades Respiratorias (INER) in Mexico City, the International Union Against Tuberculosis and Lung Disease, the Asociación Latinoamericana de Tórax, and the European Respiratory Society (ERS/ALAT SinTB project). The INER, as the national reference centre for TB, receives mostly uninsured patients from several Mexican states, the majority from Mexico City and neighbouring states.
This is a retrospective study based on a review of the clinical charts of drug resistant pulmonary TB patients monitored at the INER's tuberculosis clinic; therefore no special approval by the institutional ethics committee was required. The study was not interventional, and confidentiality was ensured.
In Mexico, culture and drug susceptibility tests (DST) are only performed in patients suspected of having drug-resistant TB, e.g. patients with a history of previous treatment. Mycobacterial culture and DST are carried out at national reference laboratories, including the INER Clinical Microbiology Laboratory (which belongs to the network of World Health Organization (WHO) reference Laboratories).
All pulmonary samples were decontaminated by the modified Petroff method and were grown on Löwenstein-Jensen medium and in BACTEC-960 Mycobacterial Growth Indicator Tubes (MGIT). Identification was made using molecular methods and DST was performed using the following doses: isoniazid (0.1μg/ml and 0.4μg/ml); rifampicin (1.0μg/ml); ethambutol (5.0μg/ml); streptomycin (1.0μg/ml), and pyrazinamide (100.0μg/ml). After 2013, all samples resistant to, at least, rifampicin (RR-TB) were also tested for the following second-line drugs: amikacin (1.0μg/ml); kanamycin (2.5μg/ml); ofloxacin (2.0μg/ml), and ethionamide (5.0μg/ml), which was previously performed only if requested and if the resource was available.
Once the diagnosis of RR-TB or MDR-TB was established, a pulmonary physician evaluated all patients, focussing particularly on anti-TB drug history and the presence of other co-morbidities such as DM, Human Immunodeficiency Virus (HIV) infection, and chronic kidney failure. All patients underwent blood tests as part of the routine pre-treatment assessment or during the first week of therapy. DM was defined as fasting blood glucose >126mg/dL in patients with no known history of DM; in patients with a previous history of DM, evolution and treatment type were also assessed. In addition, blood biometry, blood chemistry, glycated haemoglobin (HbAC1), thyroid-stimulating hormone (TSH) at baseline and final visits were performed.
The placement of an indwelling central venous line for intravenous (IV) drug administration was offered to all patients on admission to hospital (standard double lumen central venous line, 7 Fr, Arrow International or a peripherally inserted central double lumen catheter,5 Fr Groshong, BARD Access Systems, Inc.). After discharge (2 weeks on average), treatment was administered in a primary care centre (PCC) under strict directly observed therapy (DOT).7,8 Follow-up was performed monthly during the intensive phase of treatment, and thereafter every 2 months until treatment completion (20–24 months). At each visit, blood tests were requested to assess AE and a sputum sample for culture was obtained to monitor treatment. DST was repeated only if the patients did not convert culture after 6 months of treatment.
All treatment regimens were individualized and based on WHO and Mexican guidelines,9–12 the patient's anti-TB drug history, and the M. tuberculosis culture and DST results. Each regimen included at least 4 active drugs. Drug was considered to be active on the basis of DST results coupled with evidence that the patient had not taken the drug for 30 days or more. The regimens always included at least 1 fluoroquinolone (ofloxacin, levofloxacin, or moxifloxacin), 1 second-line injectable drug (amikacin, kanamycin, capreomycin), and 1 of the former WHO group 413–15 (prothionamide, cycloserine, para-aminosalicylic acid [PAS]) or group 516 (linezolid, amoxicillin/clavulanate, and high-dose isoniazid) drugs, if necessary. The prescription of each drug was based on the patient's body weight and the presence of co-morbidities such as DM, chronic kidney failure, and a history of central nervous system or psychiatric disorders. All drugs were provided by the National Tuberculosis Programme (NTP) and were administered from Monday to Saturday at the PCC. All patients were prescribed pyridoxine (at least 200mg), and other ancillary drugs were administered only if needed.
Statistical AnalysisWe conducted a bivariate analysis of variables considered either categorical or numerical, according to their distribution. Variables with a significant association with adverse events or outcomes were considered for a multivariate logistic regression analysis that included age, gender, HIV status, arterial hypertension, malnutrition and alcoholism. All analyses were performed using the STATA statistical software package, version 9.0 (StataCorp LP, College Station, TX, USA).
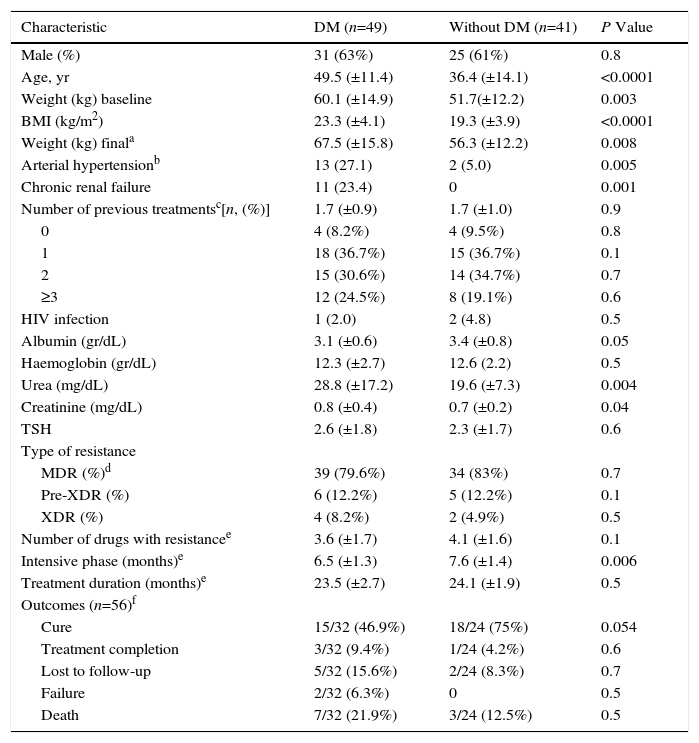
ResultsFrom 2010 to 2015, we identified 90 patients with drug-resistant pulmonary TB: 73 (81.1%) patients were identified as RR-TB (1 case) or MDR-TB (72 cases), 11 (12.2%) as pre-XDR-TB (10 samples with MDR-TB and additional resistance to a fluoroquinolone and 1 with additional resistance to a second-line injectable drug), while 6 (6.7%) patients were diagnosed as XDR-TB. Eighty-nine patients were diagnosed by culture and DST; in only one case, the diagnosis was established by Xpert® MTB/RIF, showing RR M. tuberculosis (a culture sample could not be obtained for this patient). We could only assess DST to all second-line drugs in 71/90 (79%) patients because of the limited availability of these tests in Mexico. Of the total study sample, 8 (9%) patients were treatment-naïve (5 being close contacts of an MDR-TB patient), while the remaining 82 had previously been treated. The characteristics of patients with and without DM are summarized in Table 1. The pattern of resistance was similar in DM and non-DM patients.
General Characteristics of Patients With Tuberculosis (TB) With or Without Diabetes Mellitus (DM).
| Characteristic | DM (n=49) | Without DM (n=41) | P Value |
|---|---|---|---|
| Male (%) | 31 (63%) | 25 (61%) | 0.8 |
| Age, yr | 49.5 (±11.4) | 36.4 (±14.1) | <0.0001 |
| Weight (kg) baseline | 60.1 (±14.9) | 51.7(±12.2) | 0.003 |
| BMI (kg/m2) | 23.3 (±4.1) | 19.3 (±3.9) | <0.0001 |
| Weight (kg) finala | 67.5 (±15.8) | 56.3 (±12.2) | 0.008 |
| Arterial hypertensionb | 13 (27.1) | 2 (5.0) | 0.005 |
| Chronic renal failure | 11 (23.4) | 0 | 0.001 |
| Number of previous treatmentsc[n, (%)] | 1.7 (±0.9) | 1.7 (±1.0) | 0.9 |
| 0 | 4 (8.2%) | 4 (9.5%) | 0.8 |
| 1 | 18 (36.7%) | 15 (36.7%) | 0.1 |
| 2 | 15 (30.6%) | 14 (34.7%) | 0.7 |
| ≥3 | 12 (24.5%) | 8 (19.1%) | 0.6 |
| HIV infection | 1 (2.0) | 2 (4.8) | 0.5 |
| Albumin (gr/dL) | 3.1 (±0.6) | 3.4 (±0.8) | 0.05 |
| Haemoglobin (gr/dL) | 12.3 (±2.7) | 12.6 (2.2) | 0.5 |
| Urea (mg/dL) | 28.8 (±17.2) | 19.6 (±7.3) | 0.004 |
| Creatinine (mg/dL) | 0.8 (±0.4) | 0.7 (±0.2) | 0.04 |
| TSH | 2.6 (±1.8) | 2.3 (±1.7) | 0.6 |
| Type of resistance | |||
| MDR (%)d | 39 (79.6%) | 34 (83%) | 0.7 |
| Pre-XDR (%) | 6 (12.2%) | 5 (12.2%) | 0.1 |
| XDR (%) | 4 (8.2%) | 2 (4.9%) | 0.5 |
| Number of drugs with resistancee | 3.6 (±1.7) | 4.1 (±1.6) | 0.1 |
| Intensive phase (months)e | 6.5 (±1.3) | 7.6 (±1.4) | 0.006 |
| Treatment duration (months)e | 23.5 (±2.7) | 24.1 (±1.9) | 0.5 |
| Outcomes (n=56)f | |||
| Cure | 15/32 (46.9%) | 18/24 (75%) | 0.054 |
| Treatment completion | 3/32 (9.4%) | 1/24 (4.2%) | 0.6 |
| Lost to follow-up | 5/32 (15.6%) | 2/24 (8.3%) | 0.7 |
| Failure | 2/32 (6.3%) | 0 | 0.5 |
| Death | 7/32 (21.9%) | 3/24 (12.5%) | 0.5 |
Values are expressed as mean±standard Deviation (SD). BMI: Body Mass Index; DM: Diabetes Mellitus; HIV: Human Immunodeficiency Virus; MDR: Multidrug-resistant; ND=Not Done; Pre-XDR: pre-extensively drug-resistant; XTSH: Thyroid Stimulating Hormone; DR: extensively drug-resistant.
Arterial hypertension was defined as history of previous diagnosis or serial blood pressure levels of ≥140mm Hg (systolic blood pressure) or ≥90mmHg(diastolic blood pressure), or both.
At the time of this report, the study cohort comprised 77/90 patients. We excluded 13 patients (6 MDR, 4 pre-XDR, and 3 XDR) from the analysis because they refused to be treated, requested to be transferred to another programme, died, or were lost to follow-up before completing at least 1 month of treatment; 21/77 patients (27.3%)are still undergoing treatment (11 with and 10 without DM).
Treatment OutcomesAmong the 56 patients who concluded their treatment, 33/56 (59%) were cured according to the WHO definition,12 4/56 (7.1%) completed treatment, and 2/56 (3.6%) failed treatment. Seven patients discontinued treatment despite strong advice to continue (7/56, 12.5%). Ten out of 56 died during treatment (18%); while TB was the direct cause of death in 5 cases, the remaining 5 died from other causes (1 from acute complications of DM, community-acquired pneumonia, heroin overdose, and 2 from stroke). Overall, treatment was successful in 37/56 (66.1%) patients (cure plus treatment completion); there were no statistically significant differences between outcomes in DM versus non-DM patients (Table 1), although the percentage of cure was slightly higher in the non-DM group compared with DM patients (P=0.054, Fisher's exact test).
Effects of DiabetesAs expected in our population, the most frequent co-morbidity was DM. In Table 1, 49/90 (54.4%) patients with DM are compared with 41/90 (45.5%) patients without DM. In bivariate analysis, arterial hypertension was positively associated with DM (P=0.0001), as well as chronic kidney failure (P=0.006). However, after adjusting for age and gender, no association was found. Age, weight, and body mass index (BMI) were higher in patients with DM compared to patients without DM (p<0.001). In terms of laboratory findings, urea, creatinine and, of course, glucose levels differed significantly between patients with and without DM (Table 1).
Among patients with a previous history of DM, mean evolution was 11.7 years (±6.7 years), 3 patients were diagnosed when drug-resistant TB was identified, and the mean level of glycated haemoglobin was 9.5% (±2.1). Insulin was prescribed to42/49 (86%); however, glucose control was poor in the post-treatment phase (fasting blood glucose >126mg/dL). The final serum glucose level in patients concluding treatment and in those who at least completed the intensive phase of treatment at the time of this report was 175.3mg/dl (±84.3), with 8.8% (±2.3) glycated haemoglobin (although we could not assess this latter test in all patients); after treatment, glucose and glycated haemoglobin levels did not differ significantly (P=0.17 and P=0.72), respectively. The body weight increase in patients with DM was slightly higher than in patients without DM, although this was not statistically significant (6.0 (±8.5)kg vs 4.6 (±5.3)kg, respectively, P=0.51).
Although treatment regimens were not exactly similar in all patients, they were based on the same WHO10–12 and local guidelines.4 Regimens included an average of 6 drugs, irrespective of the presence or absence of DM. The regimens used included the following drugs: ofloxacin (12); levofloxacin (51); moxifloxacin (14); amikacin (36); kanamycin (4); capreomycin (37); prothionamide (67); cycloserine (62), and PAS (15). Duration of the intensive phase and full treatment were similar between patients with and without DM (Table 1).
The time-to-sputum-culture conversion was longer in patients without DM (78.3±34.4 days) than in diabetic patients (51.1±25.7 days), although this difference was not statistically significant (P=0.06).
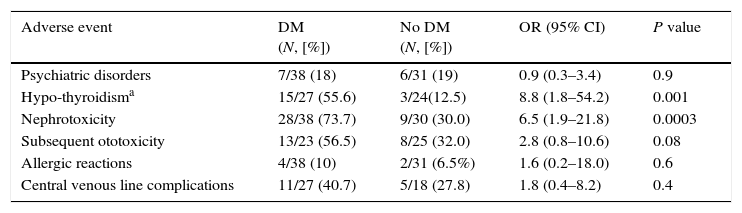
Adverse EventsThe most frequent adverse reaction was gastrointestinal intolerance; all patients reported some degree of epigastric disturbance after treatment intake, including nausea and/or vomiting, but these were easily managed and did not differ between patients with and without DM. When comparing patients with and without DM, nephrotoxicity (increase in serum creatinine of ≥0.5mg/dL (≥0.3mg/dL after 2013) hypothyroidism (TSH-thyroid-stimulating hormone ≥10μg/dl or TSH 4.5–10μg/dl if any symptoms and/or goitre) were significantly higher in the DM group (Table 2). In addition, ototoxicity was higher in patients with DM (56% vs 32% in patients without DM; OR, 2.8; [95% Confidence Interval (CI), 0.8–10.6]), but the difference was not statistically significant. Psychiatric disorders evaluated by a psychiatrist (anxiety, panic attack, suicide attempt, depression, psychosis) were documented in 13 (17%) patients; in 3 of these, cycloserine had to be stopped (no difference was found in DM vs non DM cases). We observed 6 allergic drug reactions, including 1 case of DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) associated with levofloxacin. Severity of AEs forced 7 patients to stop treatment (6 DM and 1 non-DM patient; P=0.08), in spite of efforts to improve treatment tolerance.
Frequency of Adverse Events in Multidrug-Resistant Tuberculosis Cases With or Without Diabetes Mellitus.
| Adverse event | DM (N, [%]) | No DM (N, [%]) | OR (95% CI) | P value |
|---|---|---|---|---|
| Psychiatric disorders | 7/38 (18) | 6/31 (19) | 0.9 (0.3–3.4) | 0.9 |
| Hypo-thyroidisma | 15/27 (55.6) | 3/24(12.5) | 8.8 (1.8–54.2) | 0.001 |
| Nephrotoxicity | 28/38 (73.7) | 9/30 (30.0) | 6.5 (1.9–21.8) | 0.0003 |
| Subsequent ototoxicity | 13/23 (56.5) | 8/25 (32.0) | 2.8 (0.8–10.6) | 0.08 |
| Allergic reactions | 4/38 (10) | 2/31 (6.5%) | 1.6 (0.2–18.0) | 0.6 |
| Central venous line complications | 11/27 (40.7) | 5/18 (27.8) | 1.8 (0.4–8.2) | 0.4 |
Same patients could have >1 adverse event. 95% CI: 95% Confidence Interval; DM: Diabetes Mellitus; OR: Odds Ratio.
Sixty patients accepted placement of a central venous line to receive the injectable drug. The most frequently observed AEs were the following: local infection at the insertion site, accidental removal, rupture of the line, and thrombosis. There were no significant differences in secondary AEs associated with a central line between patients with and without DM (Table 2).
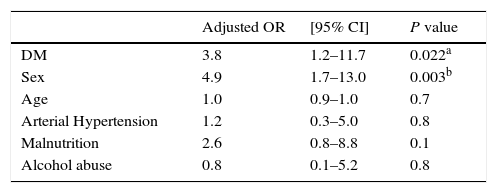
We performed a multivariate analysis for some of the adverse events and for a combination of those events requiring treatment interruption or additional treatment (nephrotoxicity, hypothyroidism, ototoxicity, or psychiatric disorders). Male gender seemed to be a risk factor for more adverse events (OR=4.9; 95% CI 1.7–14), and DM continued to be a risk factor (OR=3.7; 95% CI 1.2–11.7) after adjusting for gender, age, hypertension, malnutrition, HIV status and alcoholism (Table 3). We also performed a multivariate analysis grouping outcomes into negative (lost to follow-up, death, and failure) and positive (cure and treatment completion); DM, after adjusting for the same variables, did not have an impact on outcomes (OR 2.0; 95% CI 0.5–8.2).
Association of severe adverse events* (nephrotoxicity, hypothyroidism, ototoxicity, and psychiatric disorders) with diabetes mellitus and other patient characteristics, by multivariate analysis.
| Adjusted OR | [95% CI] | P value | |
|---|---|---|---|
| DM | 3.8 | 1.2–11.7 | 0.022a |
| Sex | 4.9 | 1.7–13.0 | 0.003b |
| Age | 1.0 | 0.9–1.0 | 0.7 |
| Arterial Hypertension | 1.2 | 0.3–5.0 | 0.8 |
| Malnutrition | 2.6 | 0.8–8.8 | 0.1 |
| Alcohol abuse | 0.8 | 0.1–5.2 | 0.8 |
CI: confidence interval; OR: Odds Ratio.
The aim of this study was to describe the outcomes of TB treatment, the impact of DM and the prevalence of AE in a cohort of patients with MDR/XDR pulmonary TB treated in the Mexican national reference centre. The main results of this study suggest the following: (a) The prevalence of DM in our cohort of TB-resistant patients is high (54.4%); (b) treatment outcomes were similarly high in both patients with and without DM, with an overall success rate of 66.1% (37/56), and (c) patients with DM had higher frequency and severity of AEs to anti-TB treatment.
The prevalence of drug-resistant TB in Mexico has gradually increased over the last 10 years5; according to the most recent survey, 2.8% (95% CI, 1.9–4) of all cases are resistant to isoniazid and rifampicin.17 Unfortunately, as in other Latin American programmes,18 notifications underestimate the true number of MDR-TB cases, as DST is not routinely performed in all TB cases.
DM is a well-known determinant of negative outcomes among TB patients, and has been associated with an increased risk of failure of primary treatment in new and/or first-line drugs in pan-susceptible pulmonary TB cases.6,19 Likewise, 54.4% of patients in our MDR-TB population had DM, and we found no difference in the prevalence of MDR-TB among new cases (8.2 vs 9.5%; P=0.07; Table 1).
In Mexico, the high prevalence of DM in the general population is mirrored in TB cases, where the prevalence of DM among TB patients is higher in comparison with other cohorts. Our results are not even comparable with those of TB patients without MDR: in Malaysia (where DM is also common) the prevalence of DM among TB patients was 26.7% (338/1267 patients).20 In a U.S. study, 42 (14%) of 297 patients with TB had DM, and Odds Ratio (OR) for death was 6.5 (95% CI, 1.1–38.0; P=0.039) in patients with DM.21 However, for reasons that are unclear, a recent study in Brazil reported a reduced mortality in diabetic patients with TB compared with non-diabetic individuals (OR 0.69; 95% CI 0.49–0.96; P=0.03).22 Although there is strong evidence of the effect of DM on the development of tuberculosis, its influence varies depending on the population studied, and therefore further investigation is needed.
Type 2 Diabetes Mellitus has been associated with the risk of MDR-TB; in a cohort of patients with TB and DM, after controlling for homelessness, HIV status, and DOT status, the relative risk of MDR-TB was calculated as 8.6 (95% CI, 3.1–23.6) in the group with DM compared with the control group (TB),23 although estimates vary in different studies. In Mexico, Jiménez-Corona et al. reported an increased risk of TB recurrence in patients with DM (Hazard Ratio [HR], 1.8; 95% CI, 1.1–2.8; p<0.05)24; the authors demonstrated by genotyping that most second episodes among patients with DM were caused by the same bacteria, although it is not clear whether there was acquired resistance.24
The use of a fixed drug combination at the programmatic level in new TB cases has helped improve treatment adherence. This, however, may not be entirely appropriate in DM patients because of the different pharmacokinetics of anti-TB drugs in this population. Different studies have described reduced serum concentrations of rifampicin and isoniazid,25,26 suggesting that these drugs should be prescribed according to body weight, as DM patients usually have higher BMI; this might explain both the negative outcomes and the higher prevalence of drug-resistant TB in this group.
In our cohort of MDR-TB and DM, all patients received second-line drugs separately, with doses adjusted according to body weight,4,10,11 although no serum levels were measured to confirm proper dosage adjustment.
This, to the best of our knowledge, is the first study comparing adverse events in a cohort of MDR-TB patients with and without DM. MDR-TB regimens require the use of multiple drugs, and therefore carry a high risk of AEs, some of which, such as neuropathy and ototoxicity, are irreversible. In our cohort, the severity of AEs varied from mild gastritis to the life-threatening DRESS syndrome, and included permanent disturbance such as aminoglycoside-related hypoacusia. AEs were common, as previously reported,27 and according to our data were more frequent in patients with DM (Table 2). This was particularly true of nephrotoxicity and hypothyroidism, and possibly ototoxicity (P=0.08), most likely due to the small number of patients included. Although we tried to improve glycaemic control during second-line TB treatment, glycated haemoglobin levels at the beginning and end of treatment were higher than expected. This clearly predisposes to the development of systemic chronic complications, and therefore, to AEs of anti-TB drugs.28
Prior to 2010, treatment of MDR-TB in Mexico was limited, due to the lack of second-line drugs. These drugs were only available at the US–Mexican border (where high incidence of DM in Mexican and Mexican-American patients was reported29) under the oversight of the U.S. authorities.30,31 In spite of the economic and programmatic limitations encountered by the Mexican NTP, the treatment success in our cohort (37/56, 66.1%), is slightly higher than that described in the different meta-analyses.32–35 Although treatment was administered in a primary care centre, it was continuously overseen by the reference centre, which could explain the slightly higher cure rate. We found no differences in treatment outcomes among DM and non-DM patients, though the cure rate seems to be slightly higher in non-DM patients (P=0.054).
The Official Mexican Guidelines36 for the treatment of TB recommend culture and DST only in patients suspected of harbouring drug-resistant isolates; the Guidelines consider DM as a risk factor for drug-resistant TB, but only in areas where DM incidence is high. However, it is not clear if the increasing prevalence of DM MDR-TB is due to late MDR diagnosis or to DM-induced changes to the pharmacokinetics of first-line drugs. Therefore, the Mexican TB Programme is considering the possibility of performing culture and DST in all cases of TB. Although the cost of this diagnostic approach is high, second-line treatment costs are even higher, being associated with increased patient disability and lower success rates.37,38 Finally, we would stress the importance of ensuring tighter glucose control in DM patients and strengthening early detection of both TB and DM (as recommended in the 2013 update of the Mexican Guidelines).36
Our study has limitations, including its retrospective nature, the relatively small sample size, the impossibility of assessing second-line DST in all samples, and the difficulty of ensuring patient adherence to the audiological and laboratory monitoring prescribed. However, the results are encouraging, given the limited resources available, and could be applied to other middle-income countries.
ConclusionsDM is a recognized risk factor for TB (and MDR-TB) infection. Although DM MDR-TB cases appear to be at an increased risk of serious treatment toxicity (such as nephrotoxicity and hypothyroidism), outcomes are similar to non-DM cases if properly managed.
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: Muñoz-Torrico M, Caminero-Luna J, Migliori GB, D’Ambrosio L, Carrillo-Alduenda JL, Villareal-Velarde H, et al. La diabetes se asocia con reacciones adversas graves en la tuberculosis multirresistente. Arch Bronconeumol. 2017;53:245–250.