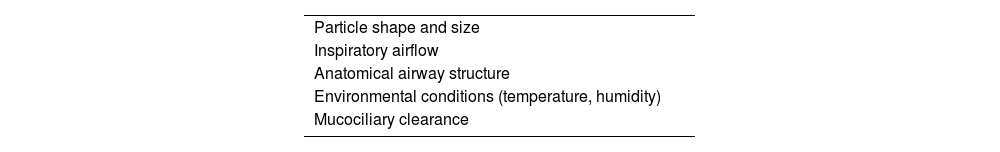
Particles suspended in the air we breathe are deposited in the airways as a function of the properties of the particle itself (shape, size and hydration), inspiratory air flow, airway anatomy, breathing environment, and mucociliary clearance. The scientific study of the deposition of inhaled particles in the airways has been conducted using traditional mathematical models and imaging techniques with particle markers. In recent years, the integration of statistical and computer methods, giving rise to a new discipline called digital microfluidics, has led to significant advances. In routine clinical practice, these studies are of great use for optimizing inhaler devices in line with particular characteristics of the drug to be inhaled and the pathology of the patient.
The air that enters the lungs contains gaseous molecules in different concentrations and particles that are suspended in this medium. Gas molecules pass through the alveolar-capillary membrane in one direction or another, depending on the partial pressure on each side, while inhaled particles are deposited along the lung walls, although the smaller molecules can also cross the anatomical barrier and penetrate the bloodstream. The exact site of particle deposition depends on their size, the speed at which they travel, their chemical composition, and their ability to adhere to the lung walls or the receptors that they encounter. The respiratory system is equipped with a set of anatomical and functional mechanisms to prevent these particles from passing to the rest of the body and to neutralize them if they do. These processes form part of the defense mechanism of the lungs that protects the whole organ system from biological (bacteria, viruses, antigens), physical (foreign bodies) and chemical (irritating gases or toxic effect) onslaught. This topic has been widely discussed in texts that are now considered reference works due to their wide perspective,1 or, more recently, in a series of magnificent reviews published in internationally renowned journals.2–4 The lungs are an excellent receptor organ for pharmacologically active particles and inhalation is an important route of administration. Drugs can be delivered using simple, manageable devices that contain therapeutic substances in solution or powder, or pre-prepared formulations. Nebulizers that transform liquids into aerosol are also available. The most commonly used drugs in the first case are bronchodilators and corticosteroids, while in the second case, nebulizers are useful for the delivery of antibiotics, antivirals and other high molecular weight substances. Anesthetic drugs are also commonly administered by inhalation.5
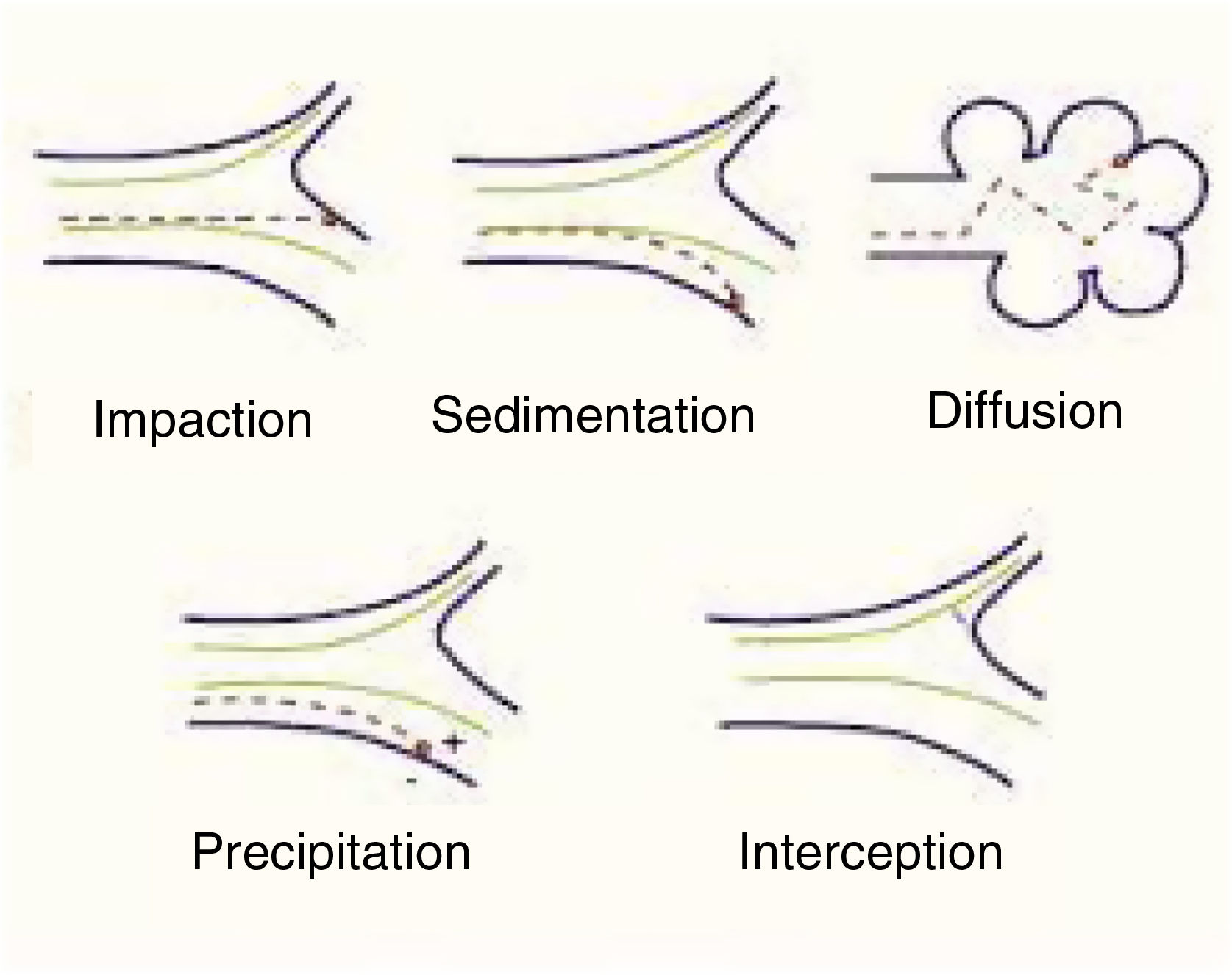
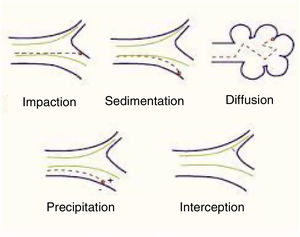
Mechanisms involved in pulmonary particle depositionThe main mechanisms of the deposition of particles in the lung are summarized below (Table 1):
Shape will help or hinder the circulation of particles within a system of branching tubes, since particles will adhere more or less readily to the bronchial walls, depending on whether they are spherical, spiked, or irregular. Some particles are inhaled in the form of complexes and aggregates, which have to be dispersed before they reach their corresponding receptors. The size of these particles will be the main factor that determines whether they reach a proximal (close to the atmosphere) or more distal (close to the alveolar-capillary membrane) territory. Size is expressed internationally as “mass median aerodynamic diameter” (MMAD), which is the diameter of a particle that has a mass equal to the median of the particles in a population, i.e., the mean diameter of the evenly distributed particles that make up the total mass of the aerosol.6 This size is expressed in microns, a convention that has given rise to the widely used nomenclature of “particulate matter” (PM), which classifies the particles according to this diameter. There are various mechanisms of deposition of these particles in the airways, as follows4–7 (Fig. 1):
- a)
Impaction or shock: Impaction occurs when the particles impact or collide violently like a projectile and are embedded in the place of contact. This phenomenon occurs mainly in the first 8–10 bronchial subdivisions, where the inhaled airflow velocity is still very high and movement is turbulent. These are mainly PM10 particles or higher, which have a greater chance of being deposited in the pharynx, larynx or upper regions of the respiratory system.
- b)
Sedimentation: Particles settle freely at whatever site they have reached when they lose their speed. They are deposited by gravity primarily in the middle and distal portions of the airway, having come to a halt when the airflow decreases. This occurs mainly in the case of PM5 particles.
- c)
Suspension: When particles are suspended, they show erratic, random movements in the most distal areas, depending on the physical and chemical characteristics of the medium, and are finally deposited or expelled as a result of respiration. The phenomenon is a result of Brownian movements, called after the author who first described them, doctor and biologist Robert Brown (1773–1858), and applies primarily to smaller particles (PM0.5). The electrostatic charges of the inhaled molecules also play a role.
- d)
Interception: Interception is a mechanism more directly related to the inhalation of fibers, which immediately stop when they come into contact with the walls of the airways. However, due to their elongated shape, particles of asbestos and certain nanotubes can penetrate to very distal territories and reach the pleural surface.
Generally, though, particles with an MMAD of 10μm or more will be deposited mainly in the oropharynx and in the upper sections of the respiratory tract. In many cases, these particles will be swallowed and will end up in the digestive tract. Particles with an MMAD of 5–10μm will be mostly deposited in the central sections of the lung and those with an MMAD of between 0.5 and 5μm will descend to the peripheral lung and the alveoli.
Inspiratory airflowThe energy needed for particles to enter the lungs is generated from the contraction of the respiratory muscles. This muscular action increases the length and width of the chest. The increase in volume creates a negative (subatmospheric) pressure that, because the airway is connected with the atmosphere, causes the intake of air. The volume of air enters at a certain speed and the particles are carried in this inspiratory flow. In the initial bronchial divisions, where the above-mentioned impaction takes place, air flow is rapid and turbulent. When the flow becomes slow and laminar, particle deposition is inversely proportional to the flow rate, since the particles are present in the airways for longer. At this stage, the main mechanism of deposition is sedimentation. Finally, in the most distal area of the lung, where the flow has practically reduced to zero, particulate movements are predominantly erratic. If the particles very small, they can even reach and penetrate the alveoli, unless they are expelled when expiration begins. The apnea recommended at the end of the inspiratory maneuver for the administration of inhaled drugs is aimed at enhancing the deposit of inhaled substances in the distal areas, fostering the adhesion of the drug to its receptors and thus improving its action. As there is no air flow in any direction, the molecules remain inside the airways for longer periods and have greater access to the above-mentioned receptors.5
Anatomical airway structureKnowledge of the tree-shaped structure of the lung with branches extending from a common trunk has facilitated the study of the circulation of particles throughout this territory.4 Various simulation models involving independent variables have been developed to define the site and magnitude of the deposit. This task, initially the area of scholars of fluidics or fluidic logic (computer science applied to fluid mechanics), has been greatly assisted by the ongoing modernization of imaging techniques. Airway diseases, some of which are very common (e.g., asthma, chronic obstructive pulmonary disease), modify the structure of the lung, either by distorting the bronchi or obstructing the airways (increased mucus, contraction), making it difficult to determine the specific characteristics of the particle deposits. One of the main consequences of these diseases is reduced expiratory flow, so the time that the particles spend in the lung and their effects are also modified, with consequent increases in both harmful and beneficial outcomes, as the case may be.8
Environmental conditionsThe environmental conditions in which breathing occurs also play an important role in particle deposition. One of the most important characteristics to take into account is the level of humidity. Particles increase or decrease in size as a function of their hygroscopicity, and this, as we have seen, determines their site of deposition. Humidity levels within the bronchial territory can also favor or prevent the adhesion of particles. This may be of great interest for the delivery of some drugs, since adhesion may be easier to achieve with particles with an appropriate MMAD for the target receptors.9
Mucociliary clearanceOne of the main defense systems of the respiratory system against the invasion of foreign substances, whether biologically active or inactive particles, is “mucociliary clearance”. Firstly, the integrity of the pseudostratified epithelium of the bronchial tree with its strong intercellular union prevents the penetration of this material into the submucosa, an innervated and intensely vascularized area that, if breached, will facilitate deeper penetration. The particulate matter that has penetrated via the lungs is trapped in the mucus that is constantly secreted primarily by the glands and also by some cells, and mobilized by the ciliary beat pattern. This movement is inward in the nasal areas and upward toward the glottis in the bronchial areas, where mucus and particles converge and are then expelled, either by swallowing or coughing. Depending on the degree of solubility of the particles (whether water or fat-soluble) they will move in one or another direction until they are absorbed or eliminated. Those that reach the alveolar territory will either be eliminated by phagocytes or will induce some pathological modifications in this hematogenous environment.10
Methods for the study of pulmonary particle depositionMethods for the evaluation of the pulmonary deposition of inhaled particles are derived from the application of mathematical models in fluid dynamics, and are classified as empirical, deterministic, stochastic, computational, and radioisotopic. All these approaches are based on a pulmonary model described by Weibel in 1963.11 Empirical models are adapted from algebraic formulations obtained from experimental observations, generally of nasal, oral or laryngeal depositions. A reference model has been developed by the International Commission on Radiological Protection12 on the basis of theoretical data, particle characteristics, and ventilatory conditions. These models are used as a reference for other forms of calculation. Deterministic models are based on simulation methods widely used in engineering that study how air flows through a tube that branches successively. The calculations take into account whether the flow is laminar or turbulent, but not the shape or size of the inhaled particles. One of the most widely used deterministic models was proposed by researchers from the University of North Carolina,13 and a recent review of this strategy found that it correlated well with experimental observations.14 Stochastic models are based on a random distribution of inhaled particles inside the airways, and are widely used to analyze the deposit of cigarette smoke and diesel particles in both animal models and humans. Calculations take into account the anatomical structure of the lung, including bronchial length and diameters, angles of division, and the cross-sectional area. Computational models, known as computational fluid dynamics (CFD), use the calculating capacity of modern computers to simulate the movement of inhaled particles. The theoretical basis of these models is the Navier–Stokes equation for the study of fluid dynamics, proposed by Frenchman Claude Navier and Irishman George Stokes more than a century ago. These calculations are so precise that they can analyze the behavior of each particle according to its velocity and pressure at a given moment and place it in any point in space. The data obtained have been validated against those of experimental studies and are currently used to determine the distribution of different particles in the lungs.15,16 However, the most commonly used method to assess drug distribution is scintigraphy and single photon emission computed tomography (SPECT), quantified from 2- or 3-dimensional images that assess the distribution of radioisotope-labeled molecules (technetium-99m, xenon-133, carbon-11 or fluor-18).17
Pulmonary drug deposition: practical considerationsThe most interesting application of the methods mentioned above is the characterization of the pulmonary deposit of commonly used drug molecules. This approach is most often used in respiratory medicine, since this form of delivery offers almost immediate pharmacological action and most of the side effects of systemic administration are avoided. Numerous devices that contain the drug in either a solution or a powder are available, and the particles are sized to allow them to reach the deepest regions of the respiratory system where the main anomalies are usually located. Aerosols generated in this way, whether in “pressurized cartridges”, in powder or generated from nebulizers, are known as “heterodisperse suspensions”. In other words, not all molecules are the same size, although their Gaussian distribution will show that the average size is that expected to be the most suitable for penetration and deposition. Therefore, the deposition mechanisms and also the way in which inspiratory flow or apnea is generated must be taken into account, as these factors are essential for facilitating drug sedimentation.18
Before being marketed, not only the drugs but also their dispensing devices must be tested using any of the above-mentioned methods in order to certify the theoretical conditions of their proposed use. Prescriptions must take into account not only the most appropriate drug but also the characteristics of the patient (age, physical limitations, learning ability, etc.) and emphasis must be placed on checking the correct use of the devices. There is no single ideal delivery system for inhaled drugs and many different commercial models are available. Climate change issues mean that hydro-fluorocarbons (HFCs), used as propellants in some types of pressurized cartridges, must be replaced by other substances that do not exacerbate the greenhouse effect,19 requiring a concerted effort by all manufacturers, doctors, and users. In some countries, where specialized companies supply this type of medication, the use of these propellants is practically non-existent. In any case, the administration of dry powder aerosols is well documented and widespread, and substitutes exist for the most commonly used medications. For new substances that could benefit from nebulized administration, there are devices capable of producing aerosols in different ways, which are simple to use, economical, and exploit the advantages of this route. Sandra Anderson et al.5 have recently published an excellent review of the past, present and future of inhaled medication, with an exhaustive list of the drugs most used in this route.
Pressurized metered dose inhalers (pMDIs)Pressurized cartridge inhalers, also called pMDIs, are devices for the delivery of aerosol medicines that are widely used throughout the world. In Spain, about 15 million units are dispensed annually, accounting for almost 50% of all drugs available in aerosol. These pMDIs are configured to deliver a fixed dose in each maneuver by way of a precision valve that controls the output of particles measuring between 2 and 4μm.20 The device consists of a metal chamber that contains the drug in solution and a liquid propellant, previously chlorofluorocarbon (CFC) but recently replaced by hydrofluoroalkane (HFA). When activated (at room temperature and under atmospheric pressure), the propellant enters a gaseous state and carries the drug with it (Fig. 2). If activation coincides with a deep inspiration, all the contents penetrate the airways. The introduction of pMDIs was a turning point in the administration of bronchodilators21 and corticosteroids.22 Aside from the synchronization needed between patient inspiration and drug delivery – the greatest difficulty in the management of this type of inhaler23 – they are easy to use, transportable and safe. The synchronization problem has been overcome by placing spacer chambers (Fig. 2) between the device and the patient's mouth, a solution that is especially useful in children.24 However, a large amount of the inhaled particles is deposited in the oropharynx and only 15–20% reach the most distal bronchial territory.25 Another drawback of these devices is the “cold-Freon” effect, more marked with CFC propellants than with HFA,26 in which inspiration is interrupted when cold particles hit the pharynx. This effect is significantly reduced with the use of spacers. In recent years HFA propellants, particularly HFA134a, that have less effect on the greenhouse gases in the Earth's atmosphere, have been used. The introduction of smaller particles (MMAD 1–2μm), especially of some corticosteroids, has also led to a much higher pulmonary deposition, with figures reaching close to 50%.27
All national and international scientific respiratory medicine societies have distributed posters, outreach articles and all kinds of material to educate patients in pMDI inhalation maneuvers.28,29 To perform a series of very precise movements so that the drug can reach its destination, patients must be properly trained and their technique must be reviewed and corrected if necessary.23 Patients must also develop a minimum inspiratory flow of about 30L/min−2. In addition to the necessary coordination between activation and inspiration, one of the most important steps is to perform post-inspiratory apnea to facilitate drug sedimentation. In some devices (Autohaler, Easyhaler), inhalation automatically triggers activation, thus helping improve lung deposition.30,31
Global warming and the evidence on climate change have fomented opinion and instigated movements to replace MDI inhalers in recent years, as the propellants they contain still contribute to the greenhouse effect. The Spanish Agency for Medicines and Medical Devices recently published a communication32 supporting the position of the scientific societies, although it warns against immediate product hopping to dry powder inhalers.
Dry powder inhalersDry powder inhalers have been used since ancient times, although they were first developed commercially with the appearance of disodium cromoglycate,33 administered in a cartridge known as a Spinhaler. These dry powder formulations are delivered via the flow generated by a deep inspiration by the patient, thus avoiding the coordination required between pMDI activation and the inspiratory maneuver. The dose is first placed in an inlet chamber or a blister which has to be pierced, and the powder is then inhaled by suction. This form of dispensing, in which the medication came in a dosing chamber or sealed blister strips, was quickly adopted for other drugs (bronchodilators and corticosteroids). Single-dose and multi-dose systems were developed for ease of use, and their popularity increased rapidly (Turbuhaler, Accuhaler, Breezhaler). Devices of this type also avoid the use of propellants, thus gaining the support of international recommendations in the drive to avoid the accumulation of atmospheric gases with greenhouse effect. There were early reservations surrounding dry powder inhalers, as patients did not perceive the delivery of the drug and in some cases it was presumed that the term “aerosol” was synonymous with dry powder suspension. Another drawback is that moisture can cause agglomeration of the powder, so devices must always be kept in a dry environment. Nevertheless, they are absolutely airtight and it is very difficult for the substance to be exposed to the air, at least until it is released for inhalation.34
The size of the particles generated with dispensers of this kind is similar to those of pMDIs, so pulmonary penetration is very similar and, in some cases, somewhat higher, reaching 20–30% of the total aerosolized dose. They be less effective in patients who are unable to generate sufficient inspiratory flow, but at a flow of about 30L/min−2 the drug reaches its receptors and the desired effect is achieved.35 The drug is usually mixed with glucose aggregates that must be dispersed by the inspiratory maneuver so that the small particles can penetrate the bronchi.36
These dry powder inhalers have become widespread in recent years and are among those most widely used for the treatment of the most common respiratory diseases. A wide range of devices is available, ranging from single-dose systems that require the insertion of a capsule containing micronized powder to multi-dose systems that already contain the drug and deliver the calculated dose for each administration. These systems are very useful and practical for the general population. Only very young or very old people or individuals who cannot generate the adequate inspiratory flow might not benefit and should resort to another type of device.5
NebulizersA nebulizer is a device that can transform a liquid into an aerosol. There are nebulizers of different types, but the most common for medical use are the jet and ultrasonic types. Jet nebulizers are based on the effect described by Daniel Bernouilli (1700–1782) that states that when a gas circulates through a tube, reducing the diameter of the tube increases the output speed and generates a suction phenomenon by reducing the pressure. If this outlet point comes into contact with a liquid surface, particles of the liquid surface are emitted in the form of suspended droplets. Connecting this area to the patient's airway via a mask facilitates entry of the nebulized drug. In most of these devices, aerosol production is generated only during the inspiratory maneuver, thus avoiding the production of drug aerosol when it is not useful.37 Ultrasonic nebulizers use a piezoelectric vibrating mesh that oscillates at a certain frequency (usually between 2–3 million times a second) to convert the liquid surface of a reservoir into an aerosol.38 Jet nebulizers can transform any liquid in which the drug is dissolved into an aerosol, while ultrasonic systems may be less effective if the solution is more viscous.39
These devices are useful for the administration of medication without the patient cooperation required for pMDIs or dry powder inhalers, and a wide range is available on the market. However, the patient must be properly connected to the device and have a consistent breathing rate of between 6 and 8L/min. Administration can be performed at rest and with a typical respiratory rate. Large amounts of medication are wasted with these devices, as it attaches to the walls of the equipment and the tubing or escapes into the surrounding air. It has been estimated that only 10–15% of the total dose penetrates the airways.40 Deposition figures can be improved by taking deep breaths followed by apnea, in order to facilitate deposition by sedimentation.41 Another significant issue is that very different sized particles are produced, so the largest particles are deposited in the bottom of the device or in the mouth and pharynx of the patient, requiring rinsing to reduce oral absorption.
Aerosols can be generated in simple ways, one of which consists of generating air pressure, similar to the jet effect, by applying simple manual pressure to a rubber bulb pump that is in contact with the liquid surface. If a simple nozzle is placed at the outlet of the container, the liquid in the reservoir can be inhaled once it is converted into an aerosol. The most widely used model is the DeVilbiss, which is easy to use, simple, and economical. Another method of aerosolizing medication is the Respimat system, patented by Boehringer Ingelheim, which consists of a suspension of “soft mist” obtained by the mechanical energy generated by a spring that compresses the drug. The suspension passes through a filter with more than 1000 perforations that gently releases the aerosol which is then simply inhaled as the patient breathes. The particles obtained oscillate between 2 and 5μm, so lung deposition can be very high, in many cases higher than the emitted 50%.42
Therapeutic possibilities of aerosolsThere are many ways of administering medications by aerosol and numerous presentations, either in a format where the dispenser and the drug are contained in the same device (pMDI or dry powder inhaler), or separately, where a separate system must be used to generate the aerosol usually in the form of a commercial nebulizer. A very wide range of beta-2 adrenergic agonists bronchodilators is available (adrenaline, salbutamol, terbutaline, levalbuterol, fenoterol, formoterol, indacaterol, olodaterol, and salmeterol), including short-acting beta-agonists (SABA) or long-acting beta-agonists (LABA) with sustained action. Another group of bronchodilator drugs are muscarinic receptor antagonists (ipratropium, tiotropium, glycopyrronium, aclidinium, and umeclidinium). These are also classified as short- or long-acting, and are widely used, especially in COPD. Another highly important strategy in the treatment of asthma is the use of inhaled corticosteroids. A wide range of possibilities is also available in this field (beclomethasone, budesonide, ciclesonide, flunisolide, fluticasone furoate, fluticasone propionate, and mometasone furoate), which cover both short-term and long-term needs, and can be used as single agents or in combination with the different bronchodilators mentioned above. Different groups of bronchodilators can also be combined. In the area of the chromones, we have sodium cromoglicate and nedocromil, drugs that have a preventive action in asthma and other allergic processes. Anti-infective preparations include broad-spectrum antibiotics (aztreonam, levofloxacin, colistin, and amikacin). Neuraminidase inhibitors (zanamivir and laninamivir), ribavirin as a treatment for respiratory syncytial virus, and pentamidine for Pneumocystis jirovecii are also available for inhalation. For the management of cystic fibrosis, recombinant human deoxyribonuclease (rhDNase) can be administered in aerosol using different commercial nebulizers (Marquest Acorn II, Hudson T Up-Draft II, Pari LC Jet Plus, Medic-Aid Sidestream), all of which produce particles measuring 2–5μm MMAD. Bronchial challenge tests, usually performed with inhalation of methacholine, histamine, adenosine or carbachol, can also be performed using a commercial powder preparation of mannitol. Inhaled prostacyclin can be used for the treatment of pulmonary arterial hypertension, and pulmonary surfactant in instillation can also be used in surfactant-deficient respiratory distress syndrome, Finally, a wide range of other substances can be administered by inhalation (nicotine in different formats, loxapine and levodopa). Insulin was used in some trials with good initial success but subsequently withdrawn due to various side effects.
Several pharmaceutical companies are testing ways of administering inhaled drugs to treat different diseases. These include cytosines (GM-CSF), chemoprotective agents such as tretinoin, interferon Beta1a, antiparasitic agents such as ivermectin, antifungals such as itraconazole and voriconazole, antibiotics such as teicoplanin, antifibrotics such as pirfenidone, and a wide variety of drugs with multiple activities.5 In Spain, a national register of these indications43 is maintained, and a rapid advance in identifying new molecules or developing a better way to manage the current ones is expected. The inhalation route fulfills one of the oldest aphorisms of medicine: to act effectively and with the least harm possible. Since the doses are low and directly target their point of action, the risk-benefit ratio is optimal in most circumstances. For this reason, the true key to the success or failure of the indicated treatment lies in following the indications for appropriate use to the letter (Table 2).
Essential indications for the proper use of inhalers.
| pMDI |
| 1. Prepare the device and place it in an upright position |
| 2. Remove the cap and shake properly |
| 3. Empty the lungs of air |
| 4. Place the dispenser in your mouth |
| 5. Start the inspiration maneuver |
| 6. Press the inhaler and continue to steadily take in air (simultaneously) |
| 7. Breath-triggered devices will be activated directly with the maneuver |
| 8. Hold breath for about 5–10s |
| 9. Exhale slowly |
| 10. Store the dispenser and if necessary rinse the mouthpiece with water |
| MDI+spacer |
| 1. Prepare the device, remove the cap, shake it, and connect it to one end of the spacer |
| 2. Empty your lungs of air and place the other end of the spacer in your mouth |
| 3. Spray the puffs indicated by your prescription into the spacer |
| 4. Breath in slowly and constantly 4–5 times |
| 5. Hold breath for about 5–10s |
| 6. Exhale slowly |
| 7. Replace the cap and store the device and clean the spacer as indicated |
| Dry powder inhalers |
| 1. Prepare the device and place it in the indicated position in front of your mouth |
| 2. Load the dose, according to the device (capsule, rotation, blister) |
| 3. Empty the lungs of air |
| 4. Place the dispenser in your mouth |
| 5. Inhale as recommended by each device (usually quickly and deeply) |
| 6. Hold your breath for about 5–10s |
| 7. Exhale slowly |
| 8. Store the dispenser and if necessary rinse the mouthpiece with water |
The authors state that they have no conflict of interests.