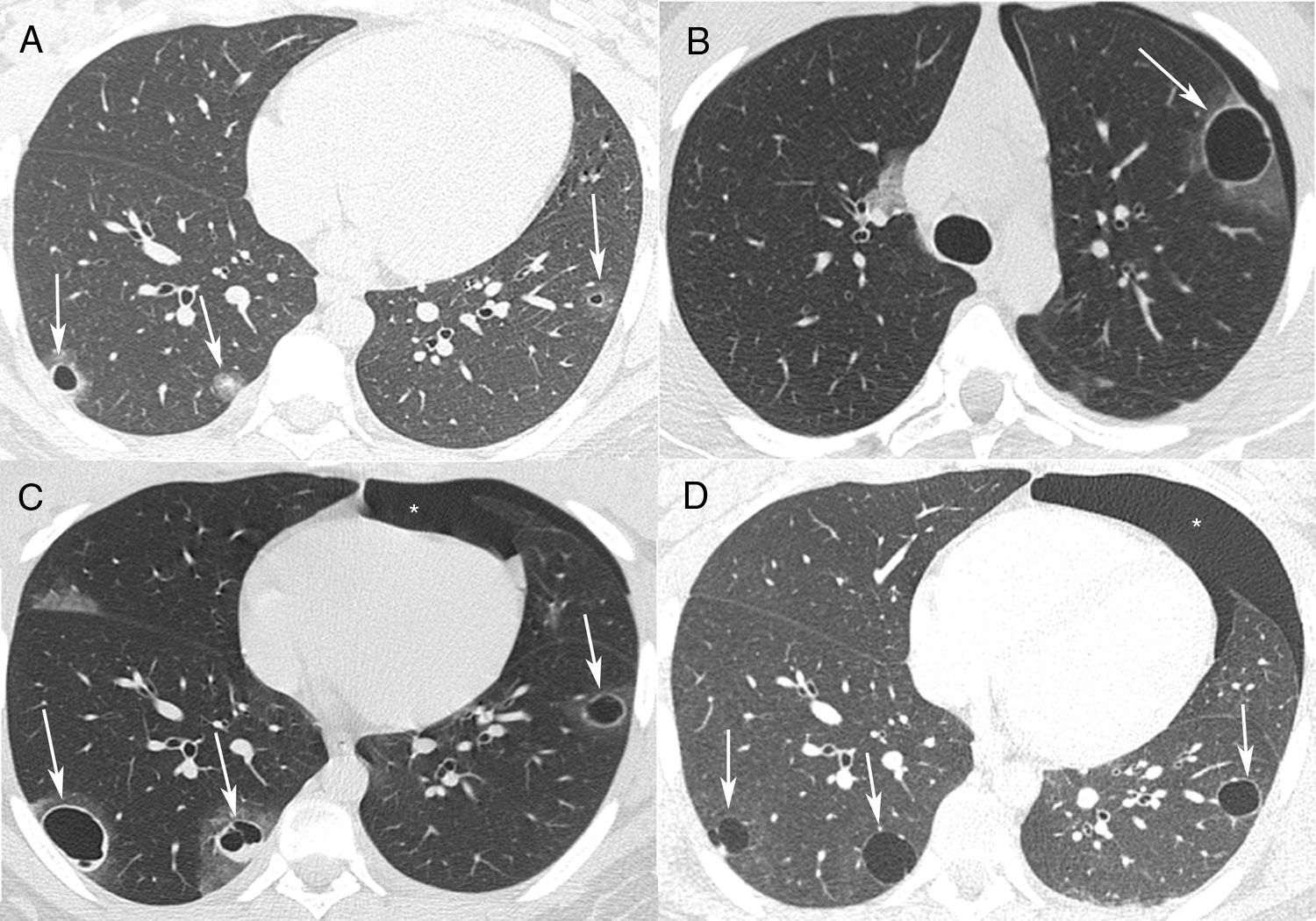
A 17-year-old male was admitted to the Emergency Department with cough and episodes of hemoptysis. The patient had a history of a giant cell tumor (GCT) in the left tibia, resected 6 months previously. Chest computed tomography (CT) revealed pulmonary nodules, some of which were cavitated (Fig. 1A). Laboratory test findings were unremarkable. The patient's sputum was negative for acid-fast bacilli. He was referred for fiberoptic bronchoscopy with bronchoalveolar lavage. The bronchoalveolar lavage fluid contained a small amount of blood, and was negative for neoplastic cells. Cultures were negative for fungus and bacteria. Video-assisted thoracoscopy was performed, and the biopsy findings from one of the nodules were compatible with GCT metastasis. The patient started a new chemotherapy cycle. Four months later, he had an episode of chest pain associated with hemoptysis. A new CT examination showed a left pneumothorax, and cavitated thick-walled nodules with ground-glass halos (Fig. 1B and C). The pneumothorax was drained. The patient evolved well, with pulmonary re-expansion. Eight months later, he had a new episode of chest pain and dyspnea. CT showed a spontaneous left pneumothorax, and evolution of the cavitated nodules into thin-walled cysts (Fig. 1D). In this phase, the patient presented metastasis to intraabdominal lymph nodes in addition to the pulmonary metastases. He underwent new chest drainage and pleuroscopy with bilateral pleurodesis through the intrapleural instillation of talc. During pleuroscopy, pleural metastases were detected. The patient underwent a chemotherapy regimen with six cycles of doxorubicin and cisplatin, which resulted in regression of some of the lung lesions. He remains in outpatient follow-up with no new complication 1 year after the last pneumothorax.
(A) Axial chest CT with pulmonary window settings shows bilateral small pulmonary nodules, two of which are cavitated (arrows). (B, C) CT performed 4 months later demonstrates a left spontaneous pneumothorax (asterisk) and growth of the nodules, which now present with relatively thick walls and ground-glass halos (arrows). (D) CT performed 1 year after A shows a new left pneumothorax (asterisk) and evolution of the cavitated nodules into thin-walled cystic lesions (arrows).
GCT in the bone is a primary intramedullary tumor; it is generally benign, but can be locally aggressive and even metastatic. Malignant transformation and distant metastasis are extremely uncommon. Malignant transformation may occur as a result of dedifferentiation of the primary tumor or secondary to previous radiation therapy. Metastasis of GCTs most commonly arises in the lungs. Pulmonary metastases are more likely to appear in patients with recurrent GCTs, and often have an indolent course; they are rarely fatal.1,2 Cavitation of metastases is extremely rare. Pulmonary metastasis initially presents as a solid mass, with an air-filled cavity formed after discharge of the necrotic material inside. The wall of a cavitated metastasis is generally thick and irregular, although thin-walled cavities can be found and may be seen with other lesions at various stages of excavation. The exact mechanism of cavitation is usually difficult to determine, but the cause is presumed to be tumor necrosis or a check-valve mechanism that develops by means of tumor infiltration into the bronchial structure. A potential complication of cystic metastases is pneumothorax, caused by necrosis of subpleural metastases producing a bronchopleural fistula.3,4 The use of pleurodesis with talc in the treatment of pneumothorax associated with cystic pulmonary metastases has been described in the literature.5,6 Authors7,8 have suggested that given the high rates of recurrence, pleurodesis should be performed after the first spontaneous pneumothorax in patients with diffuse cystic lung diseases, rather than waiting for a recurrent episode. To prevent possible right pneumothorax, our patient underwent prophylactic resection of subpleural metastatic lesions and bilateral pleurodesis. The long-term prognosis and survival rate are favorable for patients with pulmonary metastasis, except for those with sarcomatous transformation, who have a worse prognosis.1,2