Cystic fibrosis (CF) and asthma share similar symptoms and lung function (such as airflow obstruction and hyperresponsiveness). There is debate on how to best define and distinguish asthma-like features and CF-asthma overlap syndrome (CFAOS).2–4 Management of both has dramatically changed over the past years.2 Highly effective CFTR modulators, especially elexacaftor/tezacaftor/ivacaftor (ETI), are transforming CF by targeting the root cause itself.1 Furthermore, asthma biologic therapies (such as anti-interleukin [IL]-5 and anti-immunoglobulin [Ig] E monoclonal antibodies [Mab]) decrease exacerbations and improve lung function in severe asthma patients not associated with CF2 (Fig. 1).
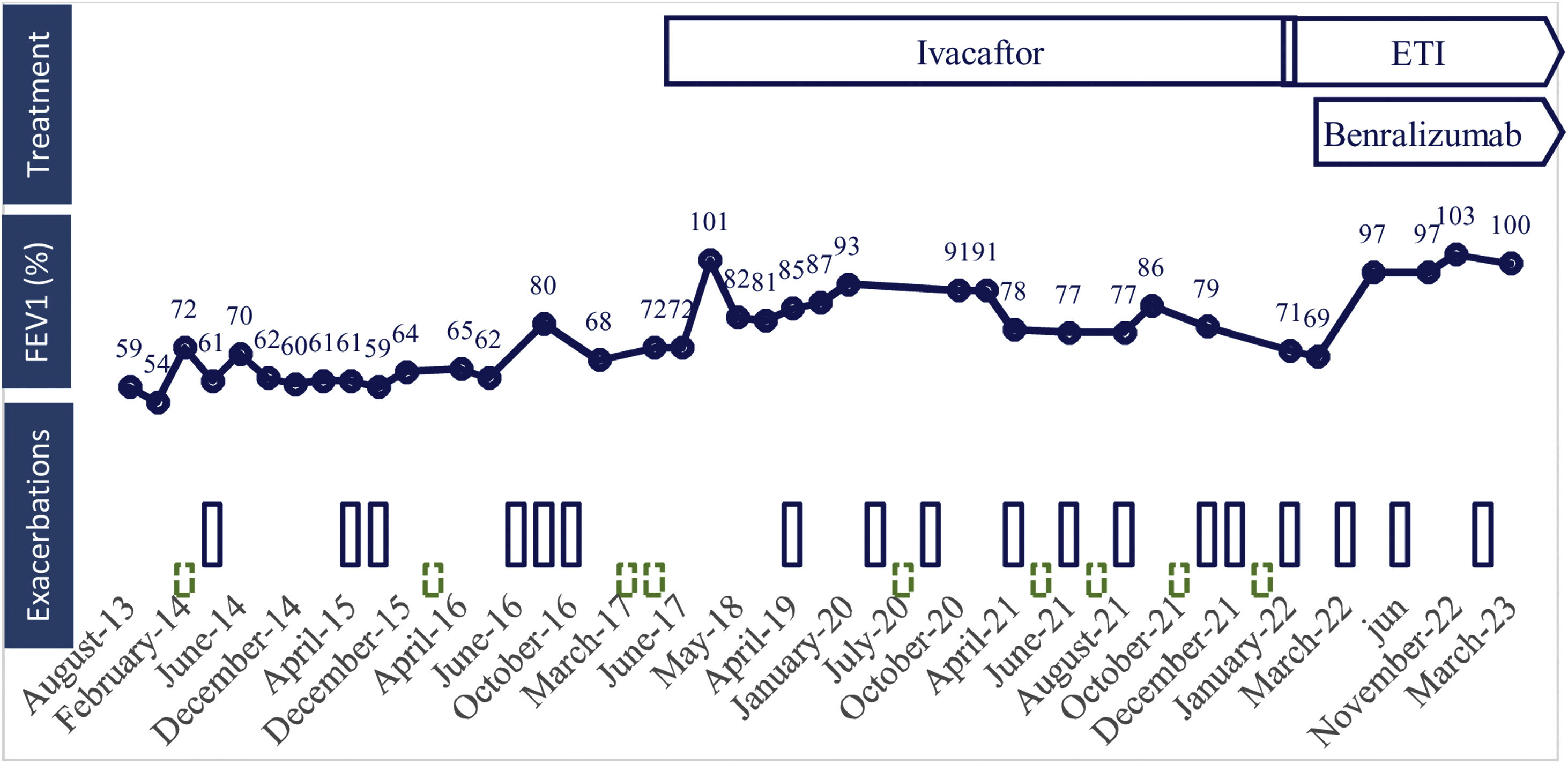
Treatment, lung function and respiratory exacerbations over time. Lung function is represented as predicted volume exhaled during the first second (FEV1 percentage). Exacerbations which required antibiotics are represented as blue bars, and those requiring corticosteroids are represented as dashed green bars. ETI: elexacaftor/tezacaftor/ivacaftor.
We report the case of a non-smoking woman diagnosed with CF (F508del/R117H) at the age of 21 (2009), studied for repeated respiratory infections, airflow obstruction and central bronchiectasis. She developed a severe respiratory involvement with bilateral cystic bronchiectasis predominantly in upper lobes with chronic bronchial infection by Staphylococcus aureus. Bronchodilators and airway clearance techniques were started. While bronchorrhea improved, she referred persistent wheezing and dyspnoea related to temperature changes, nocturnal cough and seasonal rhino-conjunctivitis. The skin prick test was positive to olive tree, Alternaria tenuis and cat epithelium. Lung function showed a moderate airflow obstruction with high variability and a high exhaled fraction of nitric oxide (FENO) of 55–103ppb. A persistent peripheral eosinophilia of 800–2300cells/μL (normal 0–500cells/μL) was observed, with persistent total IgE of 197–287KU/L (normal<120KU/L). After ruling out allergic bronchopulmonary aspergillosis, diagnosis of bronchial asthma was made. High doses of inhaled corticosteroids and long-acting bronchodilators (beta 2 agonist and anticholinergic) were initiated.
In 2017 she started ivacaftor and lung function improved. However, three years later (2020) she had a primoinfection by Pseudomonas aeruginosa treated with inhaled colistimethate sodium. Although eradication was achieved, after one year under treatment with good adherence to antibiotic and bronchodilators, (verified by electronic prescription and inhalers’ adhesion test (TAI) score 50 of 50 points), she remained with moderate pulmonary exacerbations, lung function decrease and uncontrolled asthma (asthma control test score 16 of 25 points) requiring oral antibiotics and acute oral corticosteroids.
When ETI was approved in our country (2022), we switched CTFR therapy to ETI. Mepolizumab (anti-IL5 Mab) was also started because of uncontrolled T2 (eosinophilic and allergic) severe asthma, but had to be stopped because of late bronchospasm. Later, benralizumab (anti-IL5 receptor Mab) was initiated with adequate tolerance. After one year under treatment with ETI and benralizumab, the patient had a clinical (asymptomatic, better exercise tolerance and less exacerbations), functional (resolution of airflow obstruction), radiological (bronchiectasis improvement) and microbiological (clearance of Pseudomonas aeruginosa and no other isolations) improvement.
There is no gold standard for diagnosis of CFAOS, being helpful a suggestive clinical history, presence of atopic disease, airflow reversibility with high FENO and symptoms improvement with appropriate treatment of suspected asthma.2 Treatment should be individualised. To our knowledge, this is the first case combining ETI and biological asthma treatment for CFAOS. CFTR modulators, taking into account their role on airway remodelling, may play a key role on patients with CFAOS and asthma-overlapping features of CF.3
Authors’ ContributionsAll authors contributed equally to this manuscript.
Conflict of InterestsThe authors state that they have no conflict of interests.