Cystic fibrosis (CF) is the most common severe genetic autosomal recessive disease in Caucasian populations, affecting at least 75,000 individuals worldwide.1 The disease is caused by the presence of mutations in the gene encoding for the CF transmembrane conductance regulator (CFTR) protein, a chloride and bicarbonate ion channel expressed in epithelial cells of mucus-producing organs.2 The ion transport defect results in a multisystem disease affecting the lungs, the pancreas, the intestine, the liver and reproductive organs. More than 2000 mutations in the CFTR gene have been described and are divided into functional classes based on the type of CFTR protein abnormality: class I, II, and III mutations are characterized by little or no CFTR function and are often associated with a more severe phenotype; class IV, V and VI mutations are characterized by residual CFTR function, which is often associated with a milder phenotype.2 The Phe508del allele, a class II mutation, is the most common mutation in persons with CF (pwCF): approximately 80% of pwCF have one Phe508del mutation and 40–50% of patients are homozygous for this mutation.2
Life expectancy of pwCF, which barely reached a year in 1950s, has markedly improved over the past decades with a current median age of survival over 50 years in countries with well-established CF care.2 Improved survival has been achieved due to multidisciplinary care in CF centers, pancreatic enzyme replacement and nutritional support, antibiotic therapy, airway mucus clearance and mucoactive drugs.2 Lung transplantation further offers a survival benefit in patients with advanced CF lung disease.3 As a result of improved longevity, demographic characteristics of the CF population have dramatically changed and the numbers of adults (18 years and older) exceed those of children in many countries.1 Modelling of future trends in CF demography using national registries in European countries has led to the estimation that the overall number of pwCF would increase by approximately 50%, with a stabilization in the number of children and an increase by 75% in the adult CF population between 2010 and 2025.4 Of note, these forecasts were obtained at a time when therapies directly targeting the CFTR defect were not available for most patients.
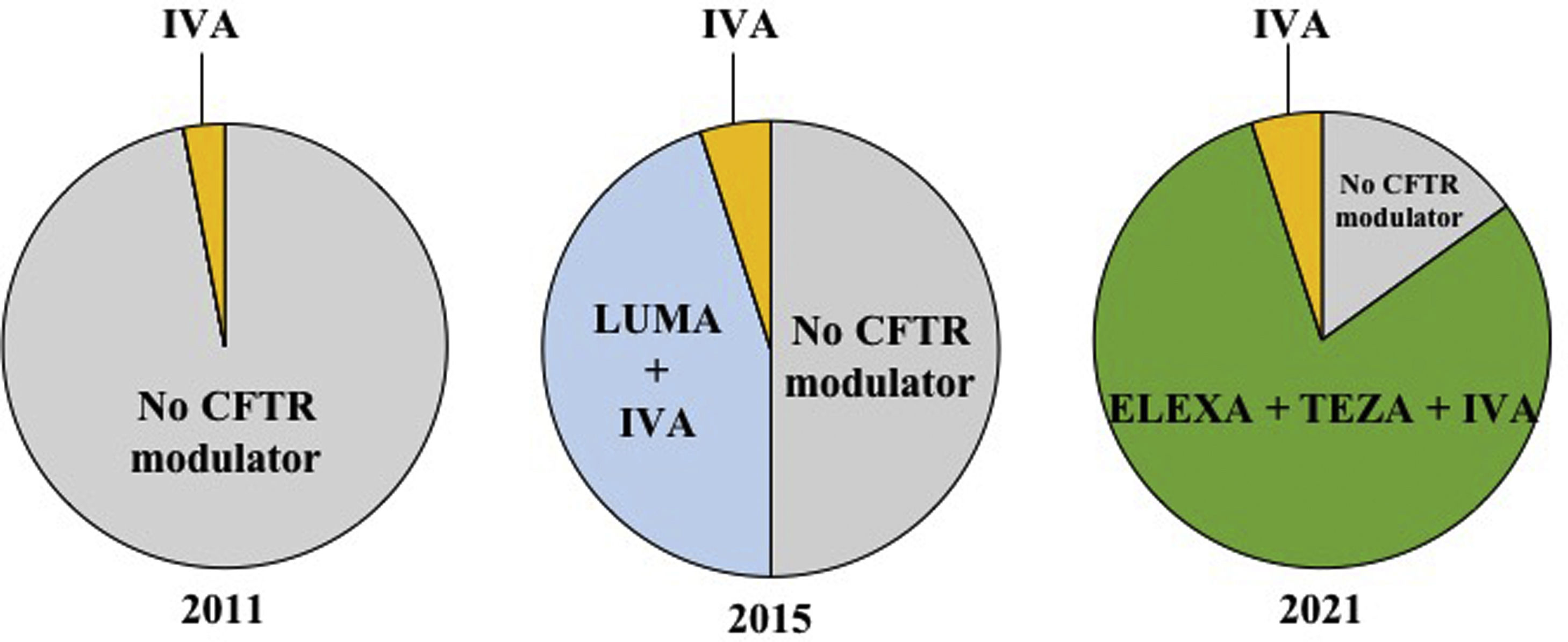
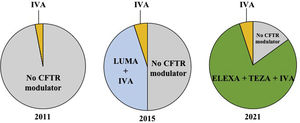
CFTR modulators are mutation-specific small molecules recently developed to bind to defective CFTR proteins and partially restore their function. Ivacaftor, a CFTR modulator that increases the CFTR channel's opening frequency and ion conductance, was the first approved for patients with gating (class III) mutations, accounting for 2 to 10% of all pwCF. In the phase 3 study, ivacaftor decreased sweat chloride, improved respiratory function and nutritional status, reduced exacerbation frequency, and improved the quality of life for patients 12 years and older.5 However, ivacaftor alone was not effective in patients homozygous for the Phe508del mutation.6 Two molecules, lumacaftor and tezacaftor, were then developed to correct the trafficking of Phe508del CFTR protein to the cell surface. The double combinations lumacaftor–ivacaftor and tezacaftor–ivacaftor for patients homozygous allowed significant clinical improvement in patients homozygous for the Phe508del mutation7,8 and tezacaftor–ivacaftor was also efficacious in patients with a Phe508del mutation associated to a residual-function mutation.9 These double combinations, however, are ineffective in patients with a Phe508del associated with a minimal function mutation (one that produces no protein and/or does not demonstrate in vitro response to modulators). More recently, the triple combination of elexacaftor–tezacaftor–ivacaftor has been shown to be highly effective for improving sweat chloride, lung function, body weight and quality of life in phase 3 studies including patients homozygous for the Phe508del mutation or a Phe508del associated with a minimal function mutation.10,11 In Europe, elexacaftor–tezacaftor–ivacaftor has been recently approved in pwCF 12 years and older with at least one Phe508del mutation. As a consequence, nearly 80% of adolescents and adults with CF are eligible to this triple therapy and approximately 90% of pwCF would be eligible to a CFTR modulator therapy. The proportion of the French CF population aged 12 years and older eligible to CFTR modulators between 2011 and 2021 is depicted in Fig. 1.
Proportion of the French CF population aged 12 years and older eligible to CFTR modulators between 2011 and 2021. Each circle chart represents the repartition of people with CF (pwCF) aged 12 and older in France, eligible for CFTR modulator therapy in 2011, 2015 and 2021. In 2011, only 3% of pwCF (with a G551D mutation) were eligible to be treated with ivacaftor, whereas 97% of pwCF had no access to CFTR modulator therapy. In 2015, approximately 5% of pwCF were eligible (with at least one gating mutation) to be treated with ivacaftor and 45% (homozygous for the Phe508del mutation) were eligible to lumacaftor–ivacaftor (patients); 50% of pwCF had no access to CFTR modulator therapy. In 2021, approximately 5% of pwCF (with at least one gating mutation) are eligible to be treated with ivacaftor and 80% are eligible to receive the elexacaftor/tezacaftor/ivacaftor combination (people with at least one Phe508del mutation); there are still 15% of pwCF who have no access to CFTR modulator therapy. Of note, patients living with lung transplantation, who represent 10% of all pwCF, are not currently eligible to CFTR modulators. IVA: ivacaftor; LUMA: lumacaftor; ELEXA: elexacaftor; TEZA: tezacaftor.
The development of CFTR modulators have raised great hope for pwCF but many questions remain to be addressed. First, the promising results obtained in highly selected populations in clinical trials need to be confirmed in real world studies that contain less selected populations. Several studies confirmed that ivacaftor is safe and effective in real-world patients, including those with advanced lung disease (i.e. percent predicted forced expiratory volume in 1s – ppFEV1 – <40), who are usually excluded from clinical trials.12,13 In a large cohort of unselected adolescents and adults carrying homozygous Phe508del mutation,14 patients who were able to continue lumacaftor–ivacaftor over 1 year had improvement in lung function and body weight and a reduction in pulmonary exacerbations that was comparable to what was observed in clinical trials.14 However, authors reported a three-fold increase in the rates of patients who discontinued lumacaftor–ivacaftor compared to the phase 3 studies (5% in clinical trials, 16% in this real-world study); rates of lumacaftor-ivacaftor discontinuation were even higher (28%) in patients with ppFEV1<40.14 Discontinuations of treatment were mostly related to respiratory adverse events that were due to lumacaftor but did not occur with tezacaftor.15 Elexacaftor–tezacaftor–ivacaftor became available in 2020 in multiple European countries in early access programs for patients with advanced lung disease. A recent study reported rapid improvement in lung function and body weight after initiation of elexacaftor–tezacaftor–ivacaftor in 245 patients with a least one Phe508del mutation and advanced CF lung disease; the safety profile was reassuring with only mild adverse effects that could be managed without drug discontinuation.16 The authors further reported a two-fold decrease in the number of lung transplantations in pwCF between 2020 and the two previous years, suggesting that triple therapy could effectively alleviate the need for lung transplantation and has the potential to improve survival in eligible patients.16
As clinical trials are often performed over a relatively short period of time (usually less than a year), they do not allow evaluating long-term effects of CFTR modulators. Open-label extension studies and real-world studies are crucial to evaluate the long-term effect of CFTR modulators. Limited data exist on the long-term effects of ivacaftor, but it is still too early to have data on the long-term effects of elexacaftor–tezacaftor–ivacaftor.
Although first tested and approved for the treatment of adolescents (12 years and older) and adults, CFTR modulators might be most effective when started early, before the onset of irreversible organ damage, and induce improvement in lung disease but also in extrapulmonary disease including pancreatic insufficiency.2 In Europe, ivacaftor is now approved for patients 6-months and older children in Europe and lumacaftor-ivacaftor is approved for patients 2 years and older. The promising results of elexacaftor–tezacaftor–ivacaftor in children 6–11 years with a Phe508del mutation will presumably lead to approval in this age group in the near future.17
There are still many situations in which unanswered questions persist regarding the benefit-risk balance of CFTR modulators. For example, only scarce evidence is available on the use of CFTR modulators during pregnancy, even though initial studies suggest that these treatments are safe for the mothers and the fetuses/infants.18 The use of modulators in specific populations, including patients with liver cirrhosis (which is present in 5–10% of pwCF) and those with solid organ (lung and/or liver and/or kidney) will also need to be explored in real-world studies. Finally, the impact of CFTR modulator on emerging complications of CF such as colorectal cancer, cardiovascular disease or chronic kidney disease are unknown and should be examined in the coming years.
In conclusion, CF care has much improved over the past 60 years, and the introduction of highly effective CFTR modulators in a growing number of patients over the past 10 years is predicted to have a profound impact on the CF population. Facing the expanding adult CF population will be challenging for CF caregivers as many questions regarding long term effects of CFTR modulators remain unanswered. Further ensuring that CFTR modulators are available to all eligible patients around the world remains a challenge as these new therapies represent important cost that may not be manageable in all countries. Despite these limitations, the CF community is currently leaving a therapeutic revolution that will benefit many patients: as Bob Dylan once wrote “The times they are A-Changin”!
Conflict of interestLR has no conflict of interest. CM has received personal fees from Chiesi and Zambon, unrelated to the present article. PRB has received research grant and personal fees from GSK and Vertex pharmaceutical unrelated to the present article. PRB has received personal fees form Astra-Zeneca, Boehringer Ingelheim, Chiesi, Insmed, Pfizer, Zambon.