Obstructive sleep apnoea (OSA) impacts approximatively one billion people globally,1,2 and is associated with detrimental cardiovascular, metabolic, and neurocognitive consequences, as well as bothersome diurnal symptoms that adversely impact daytime function and work productivity.3 It is now acknowledged that OSA is a highly heterogeneous condition encompassing various endophenotypes, defined as the association of clinical manifestations of OSA (phenotypes) and their underlying pathophysiological traits (endotypes).4 Identification of these endophenotypes is a prerequisite for risk stratification and prognosis anticipation.5
Continuous positive airway pressure (CPAP) is the first line treatment for severe OSA.3 CPAP primarily targets the anatomical endotype which is characterised by impaired upper airway (UA) anatomy and increased UA collapsibility.6 However, CPAP therapy termination rates remain high in unselected OSA populations, reaching 47.7% at three years after treatment initiation.7 At the era of precision medicine in OSA,8 identification of the specific endophenotypes of OSA provides the foundation for research and development of tailored alternatives to the current “one size fits all” CPAP treatment approach.6
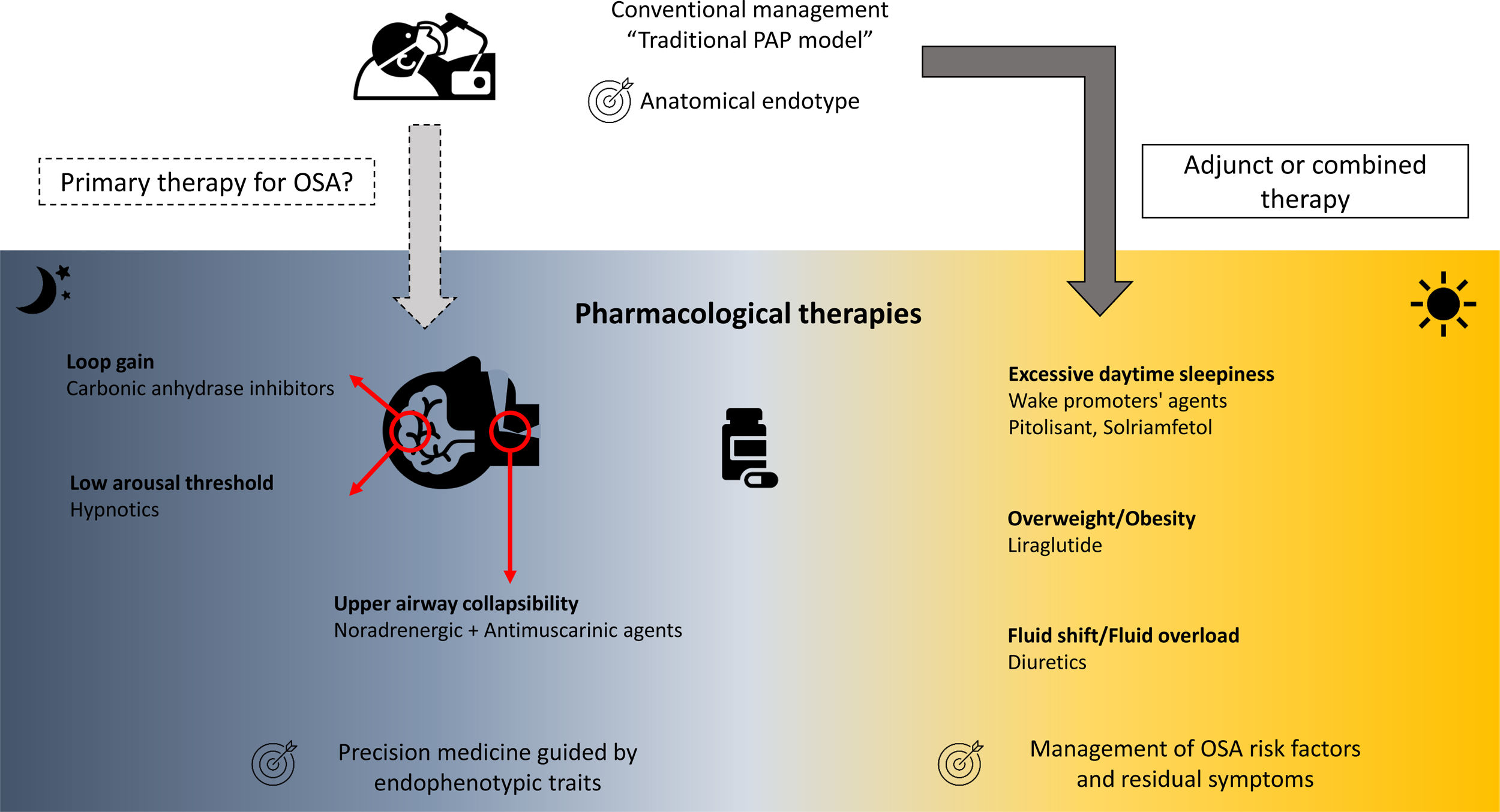
Over the last two decades, the development of pharmacological therapies for the treatment of OSA and its associated symptoms have accelerated.6 These pharmacological therapies mainly target non anatomical endophenotypic traits or “treatable traits” (i.e., reduced pharyngeal dilator function, high loop gain, low arousal threshold) existing in approximatively 70% of OSA patients (Fig. 1).6,8–10
The loss of UA dilator muscle activity at sleep onset and the lack of muscle compensation during sleep in response to UA obstruction is one of the key pathophysiological mechanisms of OSA.6,11 Current pharmacological approaches targeting UA dilator muscle activity have largely focused on combinations of noradrenergic (to compensate the lack of noradrenergic activity in non-rapid eye movement sleep participating to sleep-related hypotonia of UA muscles) and antimuscarinic (to counteract the persistent muscarinic activity involved in muscle atonia in rapid eye movement sleep) agents. Four randomised, placebo-controlled, double-blind, crossover trials to date have investigated the effects of different combinations of noradrenergic and antimuscarinic agents on OSA severity. In two short term studies conducted over a single night, the combination of Atomoxetine (Ato, a norepinephrine reuptake inhibitor, 80mg) plus Oxybutynin (antimuscarinic, broad M-subtype receptor selectivity, 5mg) or Reboxetine (4mg) plus hyoscine butylbromide (20mg) reduced the apnoea-hypopnoea index (AHI) by 63%12 or by 17±17events/h,13 respectively. Perger et al.,14 using a combination of Reboxetine (4mg) and Oxybutynin (5mg) over a one-week treatment period in 16 OSA subjects, showed a 59% median reduction in AHI, compared to a 6% median reduction under placebo. Interestingly, this reduction in OSA severity was paralleled by a significant improvement in reaction time on psychomotor vigilance test whereas no reduction in the score on the Epworth Sleepiness Scale was observed. The question of the optimal pharmacological combination and the underlying mechanism is still debated.15 The combination of Ato (80mg) plus biperiden hydrochloride (2mg, M1 muscarinic receptor selectivity) reduced perceived sleepiness whereas Ato (80mg) plus solifenacin succinate (5mg, M2 and M3 muscarinic receptor selectivity) modestly improves UA function with only a shift from obstructive apnoeas towards hypopnoeas during nonrapid eye movement sleep.15 Initial investigation of intranasal application of BAY2253651 in people with OSA, a potassium channel blocker targeting genioglossus muscle activation via pharyngeal mucosal receptor stimulation, yielded no significant therapeutic effect.16
Low arousal thresholdLow arousal threshold corresponds to the propensity to wake up too easily in response to respiratory events. This endotype may be important for 30–50% of OSA patients.17 Increasing the arousal threshold by using hypnotics can reduce OSA severity [see for review: Carter and Eckert17], challenging the common belief of the negative effects of hypnotics on UA physiology and breathing during sleep in OSA. One night of Zolpidem (10mg) increased the arousal threshold without any change in pharyngeal muscle responsiveness nor OSA severity.18 However, it increases sleep efficiency by approximately 10%, which may be beneficial in people with OSA and insomnia, the so called COMISA.19,20 The effect of adding Zolpidem (10mg) to the Ato+Oxybutynin combination (80mg and 5mg, respectively) to counteract the modest, but present, wake-promoting properties of Ato has recently been investigated.21 Zolpidem improved sleep efficiency without altering OSA severity. However, next-morning objective alertness was altered, warranting caution on the use of this combination.21
High loop gainThe unstable or excessive ventilatory responses to carbon dioxide changes during sleep is associated with an increased risk of OSA and may concern up to one-third of OSA patients.6,10 Two carbonic anhydrase inhibitors, Acetazolamide and Zonisamide, are efficacious in reducing loop gain and subsequently AHI.22,23 Interestingly, both Ato and Reboxetine may reduce ventilatory instability further reinforcing their effect on OSA severity.12,13,15 However, all these therapeutic approaches induced heterogeneous responses in terms of OSA severity reduction in unselected population. A prerequisite to their prescription would be the identification of the underlying endophenotypic traits at an individual level, which is accessible in clinical research but not disseminated in routine practice.6
Pharmacological approaches as adjunct or combined therapiesOSA risk factorsThe American Thoracic Society consensus guidelines support the deployment of add-on therapies for body weight reduction in overweight or obese CPAP-treated OSA patients,24 essentially to reduce the burden of related comorbidities. Liraglutide, a glucagon-like peptide-1 (GLP-1) agonist is approved both for the treatment of type 2 diabetes and for weight management. After 32 weeks of treatment (3mg daily) in obese patients with moderate/severe OSA, and as an adjunct to diet and exercise, significantly greater reductions in AHI, body weight, systolic blood pressure, and HbA1c were observed in comparison to placebo.25 A randomised, controlled multicentre trial (ROMANCE Study, EUDRACT No. 2014-000988-41) conducted in obese patients with comorbid type 2 diabetes and OSA, treated or not with CPAP, is investigating the effects of 26 weeks of subcutaneous liraglutide on OSA severity.26
Nocturnal rostral fluid shift is another mechanism involved in the exacerbation of OSA severity [see for review: White et al.27]. Different classes of diuretics, by targeting body fluid accumulation and fluid shift, may ameliorate OSA severity in selected patients.28
The effects of these medications targeting weight loss and fluid overload cannot be dissociated from lifestyle interventions and changes, including diet and exercise.
Residual diurnal symptomsUp to 10–15% of patients continue to experience excessive daytime sleepiness (EDS), one of the cardinal symptoms of OSA, despite appropriate management of OSA with CPAP or other primary therapies.29 Large multicentre randomised controlled trials have recently established the efficacy of solriamfetol (dopamine/norepinephrine reuptake inhibitor)30,31 and pitolisant (selective histamine receptor-3 antagonist)32,33 in reducing subjective and objective residual sleepiness. If these therapies are approved for clinical use, their long-term benefits and safety need to be established, specifically in OSA patients with cardiovascular comorbidities.
Perspectives in the next five yearsPharmacological approaches may represent an effective alternative to CPAP treatment in selected patients presenting with specific endophenotypes. However, different pharmacotherapy approaches for OSA are at different stages of maturity. No pharmacological treatment has been approved for the primary treatment of OSA so far. Long-term efficacy and safety in comorbid populations remain to be established. The large-scale deployment of pharmacological therapy requires the following issues to be addressed:
- -
Repositioning of already available drugs should be considered.
- -
In a personalised approach to treat OSA, the therapeutic strategy of choice (device- or pharmacotherapy-based) should be related to the individual specific endophenotype and risk profile. Further investigations are required to standardise OSA clusters of phenotypes for their reproducible use in future clinical trials. An automation of the identification of endophenotypes of OSA is required, for their direct clinical use, by optimising already available tools such as gold-standard polysomnography.34,35
- -
All patients may not benefit from pharmacological treatment. If these approaches may be beneficial in some, they may be ineffective or detrimental in others, especially in the long term. Cardiovascular, metabolic, and sleep-related comorbidities should be considered in the evaluation of pharmacological therapies. These steps may serve as accompanying the evolution of OSA treatment, combining precision and innovation, with the aim of delivering personalised therapies.
Sébastien Baillieul, Renaud Tamisier and Jean-Louis Pépin are supported by the French National Research Agency in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” Chairs of excellence from the Grenoble Alpes University Foundation (MIAI @ Grenoble Alpes, [ANR-19-P3IA-0003]).
Danny J. Eckert is supported by a National Health and Medical Research Council (NHMRC) of Australia Senior Research (1116942) and Leadership Fellowships (1196261). He receives Cooperative Research Centre Project Grant Funding, a research collaboration between the Australian Government, Academia and Industry (Industry partner: Oventus Medical) and has research grants supported by Bayer, Takeda, Apnimed and Invicta Medical. He serves as a consultant for Bayer and Takeda and on the scientific advisory boards for Apnimed and Invicta Medical.
These sponsors had no role in the writing of this editorial.