We report the case of a 66-year-old woman who was admitted to the pulmonology department in March 2020 for severe bilateral Covid-19 pneumonia. She had no previous respiratory history and had never smoked or been exposed to inhalants or environmental toxins. Her medical history was significant for dyslipidemia controlled by drugs, autoimmune thyroiditis that did not require treatment, and surgery for a benign breast tumor and cutaneous follicle center lymphoma with a negative extension study.
During the first 24 h after admission, her progress was poor, with clinical, radiological and blood gas deterioration despite treatment, so she was transferred to the intensive care unit (ICU). During her ICU stay, she required invasive mechanical ventilation and pronation maneuvers. Fever persisted, so antibiotic treatment (ceftriaxone, azithromycin, ceftaroline, piperacillin/tazobactam) was optimized and the Covid-19 therapeutic protocol (lopinavir/ritonavir, Betaferon® and hydroxychloroquine) was implemented. Dexamethasone was also added and a single dose of tocilizumab (400 mg) was administered. Biological parameters were significant for ferritin levels >15,000 ng/mL and IL-6 prior to tocilizumab 206 pg/mL. Finally, after a prolonged stay in the ICU, the patient improved and was discharged to the hospital ward.
Her subsequent respiratory progress was slow but favorable. Clinical improvement was associated with a progressive reduction in supplementary oxygen needs and improvement in all inflammatory parameters (PCR, LDH, ferritin, IL-6; CPK, D-dimer) and radiological infiltrates. At this time, we decided to initiate an inpatient rehabilitation program, progressively testing tolerance to sedation after prolonged admission.
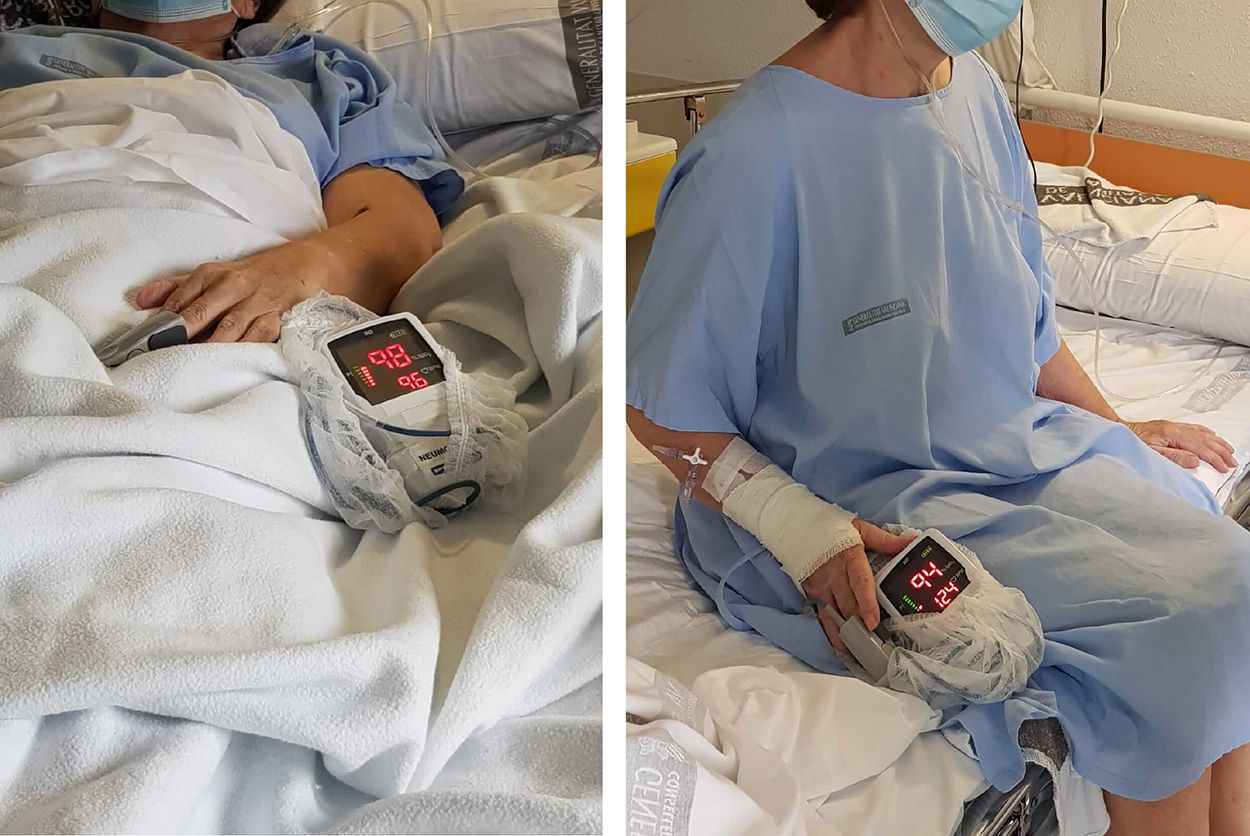
However, despite improvement, the patient presented desaturation (up to SpO2 90% with FiO2 50%) coinciding with sedation, along with tachycardia, tachypnea, distal cyanosis, and intense dyspnea, whereas this clinical picture resolved completely when the patient was returned to decubitus (Fig. 1). The first episode of desaturation was accompanied by an intense syncopal syndrome, so an urgent study was requested to rule out an acute cardiac episode (the ECG showed a known right bundle branch block, while NTproBNP and ultrasensitive troponins were normal); CT angiogram was negative for pulmonary thromboembolism and deep vein thrombosis of the lower limbs. However, CT revealed numerous areas of ground glass opacity mainly in the subpleural region, especially abundant in the pulmonary bases, all associated with the resolving infectious process. In view of the clinical suspicion of platypnea-orthodeoxia syndrome (POS), an echocardiogram was requested with intravenous injection of agitated serum that showed no signs of intracardiac shunt or delayed passage of bubbles. POS finally resolved after several weeks of convalescence.
The pathophysiological factor underlying hypoxemia in POS is the shunt effect.1 The causes associated with POS are classified as intracardiac and extracardiac (pulmonary) etiologies and a third heterogeneous group.1 In lung diseases, shunt can be caused either by the mixture of arterial and venous blood (as in arteriovenous fistulas), or by a serious alteration of the V/Q ratio,2 such as occurs in some parenchymal diseases. Gravity increases blood flow to the pulmonary bases, while perfusion pressure decreases in apical regions (dead space effect in the apex). This vascular redistribution contributes to increasing the differences in the V/Q ratio that are especially noticeable in a standing position.3 Thus, the development of POS associated with emphysema, interstitial diseases and consolidations has been described, particularly when the basal parenchyma is involved.4 Pulmonary alterations typical of adult acute respiratory distress syndrome (ARDS) would also be included within this definition.
The data analyzed so far suggest that the new coronavirus demonstrates particular tropism for the vascular endothelium. SARS-CoV-2 initiates cellular infection by binding to the angiotensin-converting enzyme receptor II that is widely distributed throughout the body, including the endothelium. Some autopsies have shown viral inclusions in endothelial cells with accumulations of inflammatory cells, findings that are suggestive of endothelitis.5 Thus, endothelial activation induced by the virus can result in both thrombotic phenomena and marked vasodilatation. In the lungs, vasodilation and endothelial dysfunction aggravate the shunt effect observed in some patients.6 In a review of radiological manifestations of Covid-19 detected on CT, dilation of pulmonary vessels, especially those closest to or within ground glass areas, was frequently observed.7 This phenomenon appears to be directly related to the production of inflammatory mediators, especially IL-1 and IL-6, cytokines that have been shown to have a potent vasodilator effect in vivo.8 Vasodilation induced by both inflammatory mediators and direct viral endothelial damage could cause the shunt effect.
The interest in this case lies not only in the rarity of POS, but in its association with SARS-CoV-2 infection. Cases of POS associated with infectious agents during the convalescence period following an episode of ARDS caused by Pseudomonas aeruginosa9 and Pneumocystis jirovecii and cytomegalovirus pneumonias10 have been reported, and it has also been described in ARDS associated with non-infectious agents such as antisynthase syndrome.11 However, no reports have appeared in the literature to date that relate POS with the anatomical and functional alterations associated with severe COVID-19 pneumonia.
Please cite this article as: Tarrasó Castillo J, Posadas Blázquez TJ, Lahosa Córdoba C, Signes-Costa J. COVID-19: enfermedad nueva, manifestaciones nuevas. Arch Bronconeumol. 2020. https://doi.org/10.1016/j.arbres.2020.07.007