Introduction
Respiratory problems are the main cause of death in some neuromuscular diseases (NMDs), and both patients and their caregivers are aware of their importance. It is thus somewhat paradoxical that, until very recently, important aspects of NMDs have remained undervalued even by specialists, including those in pneumology services. Currently, however, awareness is increasing among both neurologists and pulmonologists. Indeed, in many cases respiratory care services already provide an adequate level of care. Unfortunately, the respiratory problems in NMDs are still considered an "emerging" question in public health systems and they receive little specific funding. This situation generates clear discrimination against NMD patients in comparison with those suffering from other illnesses whose management is also difficult and costly but who already benefit from well-established social services and health care. To address current deficiencies, patient associations and pulmonologists involved in treating NMDs need to set themselves the immediate objective of establishing specialized units of the type that already enjoy a long tradition in other countries.
Justification of the Review
Some NMD patients whose respiratory muscles are already weak live without serious respiratory problems except when they need to cough. However, something as apparently banal as a common cold can demonstrate (at times dramatically) the enormous fragility of these patients by precipitating respiratory failure if they are unable to expel their secretions on their own or if they fail to receive help in doing so. The present article aims to describe both the characteristics of problems related to coughing in patients with NMD and current procedures for either helping or substituting the respiratory muscles to allow them to achieve effective expectoration, whether the main objective is to prolong life or only to attempt (at a patient's explicit request) to alleviate discomfort. In both circumstances, we must bear in mind that, unlike conventional palliative care, respiratory muscle assistance can certainly prolong life. This reality will also oblige us to be prepared to face anew the problems that accompany the task of maintaining life.
A review of the literature reveals very few studies whose design and development can be considered optimal, but the information available is of sufficient quality to establish correct guidelines for clinical practice that a posteriori analysis will allow us to improve. No patient that might be referred to us in need of these procedures should be deprived of them, and this presents an inherent problem of methodology, as the design of case studies and controls is therefore clearly further complicated. Noninvasive ventilation (NIV) in subjects with kyphoscoliosis is a prime example of such a situation: it is the preferred treatment to combat hypoventilation in these patients even though no conventionally established evidence is available. Quality clinical research must find a way to afford appropriately balanced ethical and scientific budgets.
Pulmonary Defense Mechanisms
Mucociliary Clearing
The lungs are permanently exposed to ambient air. To meet the inherent risk involved in this situation, these organs have an effective cleaning system known as mucociliary clearing. Due to ciliary beating, secretions produced in the airways transport possible contaminants from the peripheral airways to the main and upper airways, whence they are unconsciously swallowed. The secretions are a heterogeneous fluid made up of water (95%), electrolytes, amino acids, sugars, and macromolecules. These macromolecules form a colloidal suspension of glucoproteins,1 separated into 2 layers or phases (the periciliary or sol phase, and the upper or gel phase). Both layers are basically produced by the calciform cells, Clara cells, transudate, and submucosal glands.2 The amount of secretions generated in each part of the respiratory tract will depend both on airway surface area at that point and on the number of producing cells (which is in turn related to the surface area). In normal situations, the amount of secretion that reaches the trachea oscillates between 10 and 100 mL/day.3
The transport of secretions (mainly carried out via ciliary beating) depends on mechanical vectors in which the positive forces oppose those of inertia and friction. Because fluids are involved, the most important frictional forces are determined by rheological characteristics, interface surface tension, and adhesion between the secretions themselves and the epithelium. The relation between the viscosity and elasticity of the secretions is one of the determining factors in transport velocity. If the gel phase is in practice the only one really transported, the sol phase creates a low-resistance milieu where the cilia can beat, an environment that is essential for transport in the direction of the upper airways.4 At any point on the bronchial tree, the transport surface is determined by the inside diameter and the number of airways at this level. Moving from the center toward the periphery, diameters decrease, but the number of airways increases exponentially,5 so that the transport surface decreases proportionally. The small transport surface in the main airways (which could lead to the accumulation of secretions) is compensated in normal subjects by greater velocity,6 related to higher ciliary beat frequency.7
In normal conditions, secretion production is small and voluntary coughing is unproductive.8 However, when disease is present (such as in acute bronchitis) the molecular components change, production increases considerably, and sputa are formed from mucous, inflammatory cells, cell debris and, at times, bacteria. As a result of mucous production increase and poor ciliary response related to inflammation, ciliary clearing ceases to be capable of maintaining airway permeability, and coughing must take on a greater role in expelling excess secretions.
Cough: Causes and Mechanisms
Coughing serves 2 main functions: keeping the airways free of external elements and expelling secretions produced in excess or in poor rheological conditions. In experimental situations, involuntary coughing seems to be an exclusively vagal phenomenon, initiated by an irritation of the oropharynx, larynx, and lower respiratory tract, all of which are innervated by the vagus and its branches.9 Given the great variety of stimuli that can cause coughing, it must be assumed that the nerve receptors are multimodal (so they can respond to a variety of chemical mediators and mechanical stimuli) and that they are situated along the whole length of the respiratory tract.10-12 The only clear exception to vagal coughing is voluntary coughing, which is unique among all of the complex defense mechanisms that can voluntarily be assumed in an effective way.8
The inspiratory phase of a cough begins with a deep breath with a fully open glottis.13,14 While the volume inhaled can vary substantially, from total lung capacity to much lower volumes, large lung volumes provide the expiratory muscles with greater mechanical effectiveness to cough by achieving an optimum length/tension relationship and thus greater intrathoracic pressures.15 Furthermore, due to greater stretching, Pst is stronger and contributes to improving the expiratory phase. Deep breathing also opens the airways to prepare them for greater clearing. In the compressive phase of a cough, the glottis closes with the contraction of the cricothyroid muscles (which tense the vocal cords) and the transverse arytenoid (which, in its turn, closes the glottis properly) while the activity of the thyroarytenoid (the antagonist of the cricothyroid) and the cricoarytenoid muscles is suppressed.16 With the contraction of the expiratory muscles, the pleural and alveolar pressures rapidly increase to reach as much as 300 mm Hg.13 Glottal closure at the beginning of contraction also keeps the expiratory muscles in an advantageous length/tension relationship, with consequent high expiratory pressures.15 However, in spite of the great influence glottal closure exerts, it may not be essential for achieving an effective cough.17-20 Finally, the thyroarytenoid muscle (which relaxes the vocal cords) and the cricoarytenoid (which separates the vocal cords and dilates the glottis) are activated, and with this begins the expulsive phase, in which a bi-phasic flow of turbulent air is generated. This phase consists of a period called transient flow. This period has a first duration of 30 to 50 ms (in which the peak expiratory flow [PEF] appears, with values up to 11 L/s) and a second, longer duration (200-500 milliseconds) of less flow (3-4 L/s).17 The intensity of expiratory flow depends on the amount of air that leaves the main airways (the balance between the dynamic collapse caused by the high intra-thoracic pressure and the pressure gradient in the airways). The expulsive phase may be long, with elevated expiratory volume, or it may be interrupted, creating a series of short expiratory efforts, each of them with its compressive and expulsive phase. The cough pattern will depend on the point where the stimulus originates and on the type of receptor activated.8 During the initial part of the expulsive phase (transient flows), in addition to the peak flow it is possible to identify the peak value time21,22 or time elapsed from the beginning of the expulsive phase until the peak flow is reached (equivalent to 30-35 milliseconds and with a velocity of 300 L/s)17,23 and the cough expired volume or volume of air expelled in that period. The total expiratory volume of a normal cough approaches 2.5 L. The effectiveness of a cough depends on the peak flow. This effectiveness increases due to the lung elastic recoil pressure (Pst) and the elasticity of the main airways, although it may decrease with dynamic compression of the airways. The ability of a cough to remove secretions may improve owing to the upward direction of flow from the point of equal pressure, which increases the rate, kinetic energy, and turbulence of air that passes through the proximal airways.8 From this same perspective, if the cough consists of a series of expiratory efforts and lung volume decreases in each one of these, dynamic compression is progressively displaced to the most distal bronchi, the linings of which will progressively clear themselves of secretions.8
Alterations in Cough in NMDs
Causes
In a healthy subject, when mucociliary clearing fails and coughing is indispensable for bronchial clearing, the effectiveness of such clearing in eliminating secretions is determined by the amount of flow generated in the expulsive phase. These factors depend on the linear velocity of gas, the diameter of the segment, and dynamic compression, and they are manifested basically in the peak cough flow (PCF).24 In NMD there is a progressive decrease in vital capacity (VC), which is mainly related to muscle weakness.25 However, the decrease in VC in these patients is greater than the decrease in their respiratory muscle strength. This could be explained by the frequent associated comorbidity (scoliosis, cardiac insufficiency, sequelae in the form of bronchial and pulmonary infections).25
The point of full lung inflation is reached because of the adequate balance between Pst and the maximum negative pleural pressure the inspiratory muscles are capable of generating.25 If these muscles are weak, they do not manage to completely insufflate the lungs and thus are also unable to generate an adequate Pst, so that the static lung pressure/volume curve is cut off. This alteration is observed in patients with moderately weak muscular force. However, in patients with marked weakness and a long evolution additional alterations appear: Pst increases at any absolute lung volume, lung compliance decreases, and the Pst in functional residual capacity (FRC) is normal or reduced.25 The decrease in compliance would indicate that the elastic properties of the lung are intrinsically altered, and such decrease is a determining factor in the lung volume decreases in NMDs. From a theoretical perspective, 3 factors may affect lung compliance in such cases25,26: the presence of subclinical and heterogenous alveolar collapses, the generalized increase of superficial alveolar tension in consequence of low-volume ventilation, and the shortening and hardening of the elastic fibers of the lung because of sustained absence of "stretching" of the parenchyma.26 Since the level of FRC is normally determined by the balance in the tension forces of the lung and those of the chest, the decrease in this variable in patients with NMD could also be related to an increase in chest wall elastic recoil pressure. However, this does not seem very likely.25 Decreased FRC in patients with NMD seems explicable mainly by lack of tone in the thoracic muscles, which do not exercise a significant opposition to Pst. Along with this weakness, subjects with NMD also develop diminished chest wall compliance.25 It is reasonable to suppose that these changes depend fundamentally on increased thoracic cage stiffness arising from their persistently decreased expansion, subsequent hardening or ankylosis of different structures.
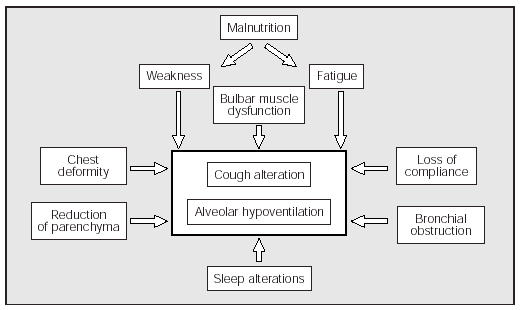
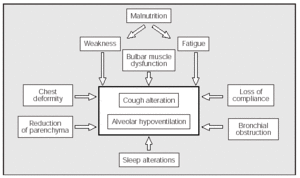
In summary we can say that the lung volume changes that appear in some NMD patients are attributable to a combination of muscle weakness and alterations of the mechanical properties of the lungs and chest wall. In the NMDs, the decrease in strength and speed of contraction of the expiratory muscles causes a decrease in the PEFs (Figure 1).
Fig. 1. Factors which, associated with the inherent weakness of the underlying disease, contribute to making coughing and alveolar ventilation more difficult in some patients with neuromuscular diseases.
Clinical Importance
Changes in the ability to cough, understood as the inability to expel secretions effectively or finding it difficult to do so, may precede alterations in alveolar ventilation. Such alterations are the main cause of morbimortality in patients with NMD.27-29 Along with hypoventilation, these alterations represent the most important problem from the patient's point of view. (See, for example, living-with-als@yahoogroups.com and vent-users@eskimo.com.)
On the other hand, the course of some NMDs leads to alveolar hypoventilation (progressive hypercapnia and hypoxemia without an increase in the alveolar-arterial oxygen gradient). In these circumstances, if mechanical ventilation is necessary, it should be administered with ambient air. Nevertheless, when acute bronchitis provokes interference in mucociliary clearing and the patient is unable to cough productively, alterations in the ventilation/perfusion relationship also appear, which worsens hypoxemia and makes oxygen therapy necessary. Acute bronchitis and mucous plugging are also the prime reasons for the appearance of atelectasis or pneumonia and may contribute to decreasing lung compliance.26 In such cases, it is useful to perform cough assist maneuvers. Paradoxically, the problem of managing secretions has received little attention in the care of NMD patients. A review of the literature shows that the functional classification of Cedarbaum30 (an instrument of reference for quantifying limitation in patients with amyotrophic lateral sclerosis) has taken respiratory alterations into account only recently31 and only in a very limited way.32,33 Even the British Thoracic Society,34 in its guidelines for noninvasive management of acute respiratory failure in these patients, focuses our attention on adequate ventilation and only contemplates some aspects of secretion management, even though such management is probably as important as ventilation itself. Moreover, in addition to the amount of secretions, their viscosity and the coexistence of upper airway obstruction should also be considered.35
Observation of standard clinical practice also reveals the inappropriateness of respiratory care. Hence, it is not uncommon to find caregivers clapping a patient on the back or extracting secretions through a nasal tube. Such practices may bring on vomiting and laryngeal spasms in addition to discomfort. Another practice of doubtful appropriateness is that of prophylactic tracheostomy carried out before it is strictly necessary. It should only be resorted to in clinical contexts in which the means for noninvasive management are unavailable or when bulbar muscle dysfunction indicates problems will develop in the near future. Although tracheostomy is indicated as a positive approach (allowing access to secretions), it is not risk free. It is a surgical intervention that generates a vicious cycle of colonization and infection and requires the availability of aspirators and catheters in the patient's home. Moreover, many complications are linked to tracheostomy. For example, keeping the cuff inflated for 4 hours may affect ciliary structure and function for up to 3 days,36 with retention and colonization of secretions and eventual formation of mucous plugs.37 Furthermore, the interference of the tracheostomy tube on certain neck muscles and the pressure it puts on the esophagus may affect swallowing, thereby possibly increasing the incidence of aspiration. Other complications that may appear are the formation of granulated tissue, tracheomalacia, hemorrhagic tracheitis38 and tracheal perforation.39 Tracheostomy requires frequent cleaning of the cannulae, care of the area around the stoma, periodic replacement of the tube, and supplementary humidification. Aspiration of secretions in tracheostomized patients, in addition to causing episodes of hypoxemia, may lead to other problems as well.39 Only proximal airway secretions are cleared and it is very difficult to access the left bronchial tree to extract very adherent secretions. Furthermore, aspiration through the cannulae occurs through small orifices at a very high negative pressure that causes repeated lesions on the wall with destruction of cilia. Secretions can be removed from tracheostomized patients much more effectively by using mechanical insufflation-exsufflation (MI-E).40
The Effectiveness of Cough
Clinical Evaluation
Medical History. The situation can vary enormously depending on a patient's medical history (chronic obstructive pulmonary disease, asthma), the phase in which the patient's condition is evaluated, and the particular NMD involved. At the positive end of the continuum will be patients who consider their cough to be satisfactory and productive. At the negative end will be those who are unable to generate even minimal coughing, either in cases of acute bronchial disease or after minor aspirations of food fragments. This situation of "loss of control" is evident both for patients and for those around them and should be considered a potential emergency.
In evaluating the circumstances related to cough effectiveness, it is useful (because of its prognostic value) to look for altered articulation of words or swallowing (both being expressions of bulbar dysfunction) as well as changes in speaking volume (related to the strength of the thoracic muscles). The ability to perform a Valsalva maneuver shows the ability to close the upper airways, which is essential both for effective spontaneous coughing and manually assisted cough maneuvers. An especially dangerous situation comes with the association of altered swallowing and the absence of effective cough, with risk of death by suffocation or aspiration pneumonia. It is also useful to include a quantification of the ability to cough35 in the medical history, for example, 4 to indicate normal cough; 3 to indicate problems during colds but in an autonomous patient; 2 to indicate need of manual assistance; 1 to indicate the patient needs mechanical assistance; and 0 to indicate the patient is unable to cough.
Regardless of how long an NMD has been progressing and of lung function values, a patient who has already had problems in the course of a common cold must begin to receive training in cough assist techniques. On the other hand, the NMDs have a natural history that can be affected by severe episodes that require immediate interventions, at times very aggressive ones. It is thus indispensable to inform a patient (and those around him or her) of the disease's pattern of progression and timing and of the various critical situations that can arise. The patient will thus be able to express a decision in regard to therapeutic procedures, particularly vis-a-vis invasive means such as tracheostomy and gastrostomy. All caregivers in the patient´s area should have easy access to a statement of the patient's decisions.
Physical Examination. Findings will also depend on the particular moment in the course of the disease. In the initial phases, the range of ventilatory movements is satisfactory, the cough maneuver normal, and the subject is able to carry out the Valsalva maneuver. Auscultation reveals no abnormal sounds. In other cases, however, simple observation shows that the patient seems exhausted. This is a reflection of great suffering due to the feeling of dyspnea and mucous accumulation, small ventilatory movements are performed with great participation of the accessory muscles and coughing efforts that are unproductive and hardly audible. Auscultation reveals the existence of abundant secretions and lack of effective mobility.
Other Tests. Oxygen saturation may be diminished, even without radiological alteration. This is explained by hypoventilation and alterations in ventilation/perfusion ratios and the shunt effect associated with atelectasis and mucous plugs.
Functional Assessment
The main aim of functional assessment is to be able to plan a preventive and therapeutic strategy that is adequate to specific needs, with actions that often begin in assisted cough training techniques for the patient and those around him or her. However, it is important to state that some of the tests and examinations discussed here cause the patient discomfort or have still not been clearly shown to have prognostic value or to be useful in guiding decisions about therapy.
1. Measurement of the Maximum Expiratory Pressure (PEmax). Because the ability to produce transient peak flows during a cough maneuver depends in large measure on the ability to generate adequate expiratory pressures, an appropriate way to determine such ability would be to measure the pressure generated by the expiratory muscles. Szeinverg et al41 evaluated those pressures in patients with dystrophies, finding that PEmax values greater than 60 cm H2O are associated with the presence of transient peak flows during the cough maneuver (effective cough). However, other authors fail to agree on the cutoff point.42 Moreover, the use of PEmax to determine the effectiveness of cough capability has limitations derived from the measurement procedure itself (inadequate cooperation, leaking).42
2. Measurement of Gastric Pressure During the Cough Maneuver (Cough Pga).42-44 The ability to achieve transient peak flows during a cough is related to abdominal muscle strength. Thus, Byrd and Hyatt44 consider that the Pga measurement is better than that of PEmax (at the mouth) for estimating expiratory muscle force in patients with pulmonary diseases. The Pga value during a cough would reflect the force generated by the expiratory muscles in the expulsive phase. Cough Pga values below 50 cm H2O do not register transient peak flows during a cough.42 The cough Pga values established as normal for adults would be greater than 175 cm H2O for men and 100 cm H2O for women.43 Although this method has been proposed by the American Thoracic Society and the European Respiratory Society,45 it is an invasive technique with technical problems, above all in patients with swallowing difficulties due to bulbar dysfunction.
3. Measurement of the Peak Cough Flow.46-48 PCF magnitude determines the ability to eliminate respiratory secretions during a cough.24 Values below 160 L/m are associated with failure in attempts to close a tracheostomy,49 and values below 270 L/m indicate a high risk of a cough being ineffective during an acute respiratory disease.28 In this case, it is imperative to begin training in assisted techniques. On the other hand, the inability to generate sufficient PCF for measurement has been related to increased mortality in patients with motor neuron disease.50 Nevertheless, the reference figures mentioned are only approximate values, since they are not based on prospective studies and have certain methodological limitations.49 In all but 1 study we examined, the figures have proved useful in clinical practice28,32,51,52 and--again except in a single study55--the figures correlate with conventional lung function and muscle function tests,53 both in healthy subjects and NMD patients.54
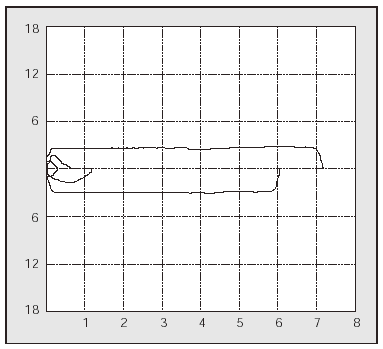
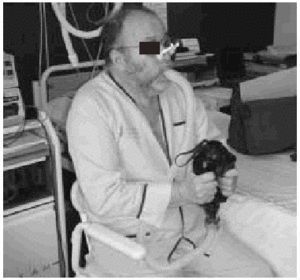
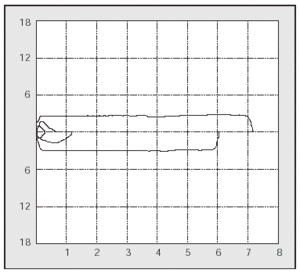
PCF can easily be determined in the outpatient clinic by portable spirometers (used also to measure PEF),56 with good agreement in the values obtained with a pneumotachometer except for very low values (Figure 2). The difference between PCF and PEF is useful for assessing the degree of bulbar dysfunction in NMD.57
Fig. 2. Peak expiratory flow measurement with a portable meter during an assisted cough maneuver performed with a patient with Becker's disease.
Respiratory infections have been found to lead to reduced respiratory muscle strength both in patients with NMD58 and in healthy subjects.59 The possible causes offered are muscular dysfunction secondary to the oxidative stress that accompanies infections, alterations in neuromuscular transmission during acute processes, and the decrease in the supply of magnesium and phosphorus in these periods.58 When severe chest wall restriction is also involved, PCF tends to be very low owing to combination of the restrictive syndrome and inability to carry out an effective expulsive movement during cough.39
4. Measurement of Maximum Insufflation Capacity (MIC). MIC is the maximum volume of air that can be held with a closed glottis and then expelled.39 MIC can be obtained by insufflating air via an inflatable bag or a volumetric ventilator, coordinating insufflations with glottic closure to keep air from escaping. It is not possible to achieve this in patients whose bulbar dysfunction prevents effective glottic closure. Patients with a MIC of less than 1500 mL have diminished spontaneous and assisted PCFs,60 which increases morbimortality.61 In clinically stable patients with motor neuron disease in whom PCF exceeds 240 L/min after MIC, mechanical cough assistance is not useful.62 To achieve flows that avoid mucous accumulation by manually assisted coughing, it is necessary to obtain a minimum MIC of 1 L.63 In motor neuron diseases, the ability to achieve a MIC that is much higher than the VC has been shown to be an essential condition for successfully maintained noninvasive management in the medium term.32,52 By the same token, the predictive value of MIC is greater than that of VC.32,52
The inability to generate a PCF greater than 2.7 L/s even when MIC exceeds 1 L, generally indicates the existence of fixed upper airway obstruction or significant bulbar impairment, with hypopharyngeal collapse during mechanical assistance to aid cough.39,62 Given that some lesions can be referred to the surgeon for corrective surgery, it is always necessary to carry out an exploration of the upper airways. Additionally, in this last situation, we have found that noninvasive methods of cough assistance will probably be ineffective (unpublished results) and other means of managing the situation should not be delayed.
5. Other Means. Mention must be made of the measurement of mucous transport through the bronchial tree by radiolabeled tracers,64 a technique that has been used above all to study mucociliary clearance, as well as the measurement of the volume of expectorated mucous,65 which in NMDs can only ld be desirable to have a more highly developed theoretical base for these procedures. Nevertheless, the absence of such a base does not justify delaying their use until more evidence is at hand.
Lung Expansion Techniques
It is striking that, in spite of the fact that hyperinflation therapy can correct acute changes in compliance,66 the only studies on the long-term effect of such treatment were carried out on patients that already had considerable lung restriction (VC<50% of predicted) and the insufflation pressures applied were insufficient (<30 cm H2O).26,67 In fact, pressures should be at least 40 cm H2O and insufflations should be done several times a day via ventilator or inflatable bag with a mouthpiece or oronasal mask. In children with muscular dystrophies, these management techniques should be carried out before their VC falls below 50% to 60% of predicted so as to minimize the alterations in compliance during growth68 and avoid their possible negative repercussion on the achievement of a sufficient MIC to obtain a good PCF in adult life.
Assisting the Respiratory Muscles
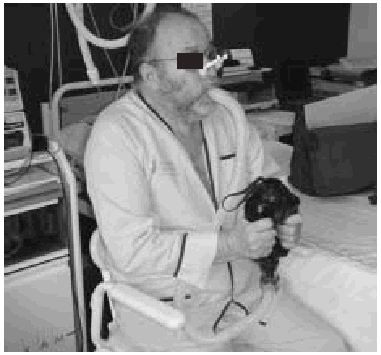
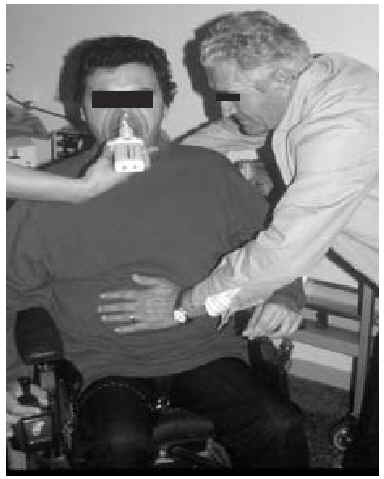
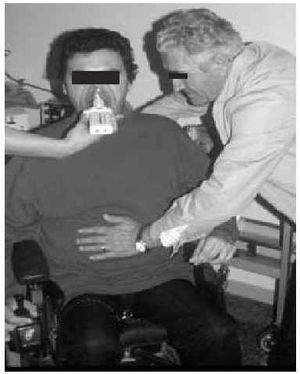
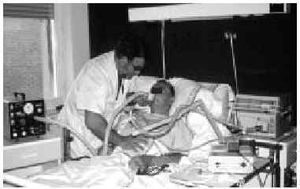
Manually Assisted Coughing. If, as has been previously stated, the inspiratory muscles of some NMD patients are incapable of deep inspiration before a cough, and the expiratory muscles lack the necessary strength to provoke an explosive discharge of air that dislodges secretions, then it is appropriate to adopt procedures that assist the work of these weakened muscles. This situation could be defined when VC is below 1000 to 1500 mL, or when PCF is below 4.5 L/s in a stable situation. Manually assisted coughing makes it possible to triple the PCF value. In patients with VC of less than 1.5 L and good bulbar function, manually assisted coughing begins with a maximum inspiration followed by the retention of air closing the glottis. The glottis will then open to allow 1 or more insufflations (with an inflatable bag or volumetric ventilator). When MIC is reached, the caregiver applies manual pressure to the thorax, the abdomen, or both via a blow coordinated with the final glottal opening and the maximum expiratory force. This technique requires a cooperative patient, with good coordination and adequate physical force. Moreover, frequent applications are necessary. Patients who have the use of their arms can themselves self-assist the maneuver (Figure 3). In general, the technique is ineffective in the presence of acute scoliosis and should be performed with caution in the presence of osteoporosis. Patients who retain a certain degree of strength in their abdominal muscles can use these muscles to generate a defense reaction against pressure, a circumstance that may neutralize or reduce the effect of the technique. Manually assisted coughing is also ineffective when MIC is not greater than VC.62 One of the aims of the physical therapist should be to train caregivers to obtain similar results. It is desirable for the training to be carried out in a stable clinical situation and prior to the beginning of cough problems. Manually assisted coughing should not be carried out until 1.5 hours after meals. Nevertheless, if choking occurs, it may help to expel any aspirated food and thus attend to the emergency situation. When manually assisted coughing does not achieve the desired result, MI-E is the best alternative for generating effective PCF and eliminating secretions.62
Fig. 3. Patient with amyotrophic lateral sclerosis without significant bulbar dysfunction carrying out insufflation maneuvers to obtain maximum insufflation capacity.
MI-E. Insufflation is forced inhalation of greater volumes than those the patient can achieve with his or her own inspiratory muscles. Exsufflation is forced expiration of greater volumes and at more rapid flow rates than those that can be obtained by the expiratory muscles. MI-E consists in providing the lungs with deep insufflation (at a pressure of 30-50 cm H2O) followed by immediate forced exsufflation (negative pressure of 30-50 cm H2O). With an inspiratory time of 2 seconds and an expiratory time of 3 seconds, there exists a very good correlation between the pressures used and the flows obtained.69 The combination of exsufflation with an abdominal or thoracic compression can increase expulsion flows68 (Figure 4). When manually assisted coughing is incapable of generating an effective PCF or when the patient is incapable of cooperating, MI-E is the most adequate option68 (Figure 5). MI-E applied with an oronasal mask can generate PCFs greater than 2.7 L/s in motor neuron disease patients, with the exception of those with very acute bulbar dysfunction,62 in whom there exists great instability of the upper airways.59 If, in normal subjects, the sudden application of negative pressure at this level produces reflex activation of the genioglossus to maintain permeability,70 in those patients with diminished strength and speed of the pharyngeal muscles71 there will be obstruction during the expiratory phase (Figure 6). Therapeutic intervention requires some 5 cycles, followed by a brief period of normal respiration (or return to the ventilator) to avoid hyperventilation. The technique should be repeated until secretions are expelled and desaturations caused by mucous plugs are corrected. During upper airway infections, exsufflations may be necessary every 10 minutes. When mucous plugs are eliminated in acute episodes in ventilated neuromuscular patients, MI-E may attain 300% improvement in VC as well as normalization of hemoglobin saturation.60 Counter indications of the technique include previous barotrauma, the existence of bullae, emphysema, or bronchial hyperreactivity. Secondary effects, such as pneumothorax, aspiration, or coughing up blood are reduced considerably by treating the aforementioned counter indications. On the other hand, gurgling noises and abdominal distension are rare and can be eliminated by lowering the insufflation pressure. The significant increase of forced expiratory flows in periods immediately following post-exsufflation indicates that MI-E does not provoke obstruction of the airways.60 As patients with spinal shock can present bradycardias, MI-E should be carried out with care in them, with gradual increase in pressures or premedication with anticholinergics. In patients with very low VC who have not previously received maximum insufflations, the use of high pressures may cause thoracic muscle discomfort; thus, progressive increase is also indicated. When patients have a PCF greater than 4.5 L/s, MI-E is not necessary since it will not increase cough flows.62
Fig. 4. Peak flow curves during cough recorded on a flow/volume axis by a conventional pneumotachometer and oronasal mask in a patient with amyotrophic lateral sclerosis. The smallest curve corresponds to a spontaneous cough, the next to a maximum insufflation capacity cough with manual assistance, and the largest to a mechanical insufflation-exsufflation maneuver. Manually assisted and, above all, mechanical insufflation-exsufflation coughs achieve a better peak flow during cough and greater mobilized air volumes.
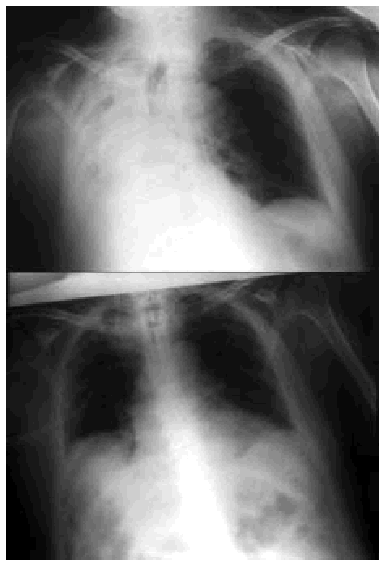
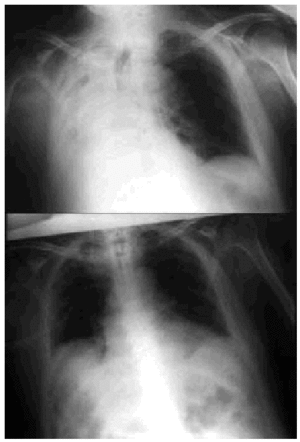
Fig. 5. Right-sided atelectasia in a patient with very advanced Alzheimer's disease who was unable to achieve an effective cough. After 6 hours of mechanical insufflation-exsufflation therapy and oronasal mask (every 15-30 minutes), and without any self-assistance, the atelectasia disappeared.
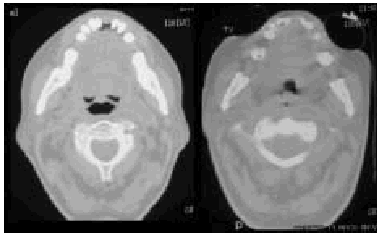
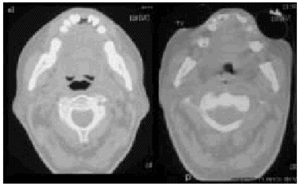
Fig. 6. Computerized axial tomography scans showing the difference inpermeability of the upper airways during an insufflation maneuver withthe Emerson Coflator and during exsufflation in a patient withamyotrophic lateral sclerosis and bulbar dysfunction.
Secretion Management and NIV. NIV can be maintained continually in patients who do not have a significant bulbar dysfunction and who prefer noninvasive treatment over ventilation via tracheostomy.28,52 However, if patients have an abnormal increase in secretions it will be impossible to continue noninvasive treatment successfully. Oxygen therapy and/or manually assisted coughing will not resolve the situation, and atelectasis and great discomfort may appear with risk of death. In these cases, MI-E may be the only effective way to substitute the action of the respiratory muscles, the coordination needed for coughing and controlling the glottis72 (Figure 7). The 80 cm H2O decrease in airway pressure generates an exsufflation with flows greater than 3 L/min69,73 as long as there is no collapse of the upper airways due to bulbar dysfunction.62 When the aim is to maintain NIV during an infection, assisted cough procedures are indispensable. However, whereas any trained caregiver can carry them out when the patient is stable, they should be performed by specialists during decompensations in order to guarantee safety and effectiveness.
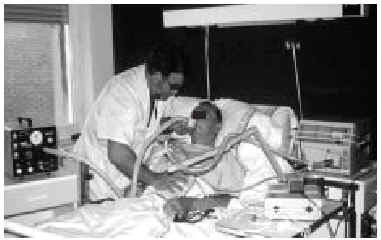
Fig. 7. Patient with amyotrophic lateral sclerosis and acute respiratory infection and ineffective cough with secretions being extracted via mechanical insufflation-exsufflation through an oronasal mask. Mechanical insufflation-exsufflation, along with alternation of different models of mask interface, allowed continual noninvasive ventilation during the decompensation episode.72
When bulbar impairment is advanced, MI-E maneuvers can be initiated, but if they are not clearly effective and fail to manage secretions adequately, the airways should be intubated immediately. This contingency must be foreseen so that the patient's authorization is available in their living will (written when the disease process is stable). There is a consensus that crises are a bad time to take this type of decision.29
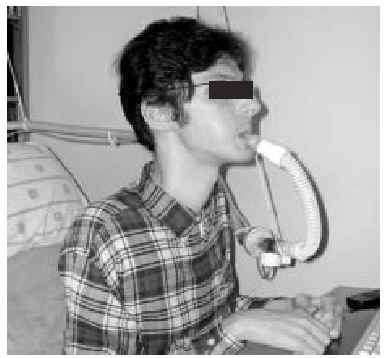
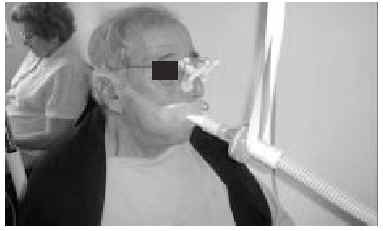
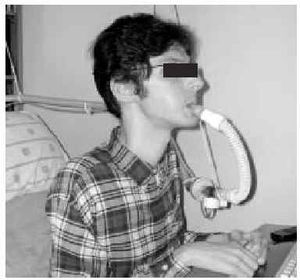
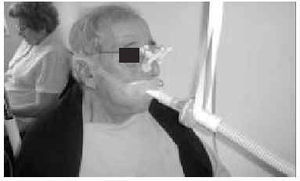
The nasal or oronasal mask must be alternated with a mouthpiece (Figure 8) when NIV is continuous (whether in situations of decompensation or stability). A mouthpiece may require a lip seal if the patient does not have the strength to compress his or her lips over the mouthpiece (Figure 9). These alternatives should be known by doctors and other health personnel who care for the patient. They represent an appropriate way of maintaining life and relieving suffering74 in patients who choose not to undergo tracheostomy.
Fig. 8. Patient with Duchenne muscular dystrophy and continuous mechanical ventilation by volumetric ventilator. For sleeping, an oronasal mask is the interface; for awake periods, a mouthpiece is used.
Fig. 9. Patient with amyotrophic lateral sclerosis who has insufficient strength to correctly close his lips around the noninvasive ventilation mouthpiece using a lip seal mask as interface during waking hours.
Correspondence: Dr. E. Servera.
Avda. Blasco Ibáñez, 84. 46021 Valencia. Spain.
E-mail: emilio.servera@uv.es
Manuscript received for publication May 5, 2003.
Accepted for publication May 25, 2003.