The most common cystic fibrosis (CF)-causing mutation is deltaF508 (F508del), which is present in 28% of CF Spanish patients. While the literature based on real-life studies on CF patients homozygous F508del treated with lumacaftor/ivacaftor is limited, it demonstrates the need for better strategies to prevent related adverse events (AEs) as well as the development of newer drugs.
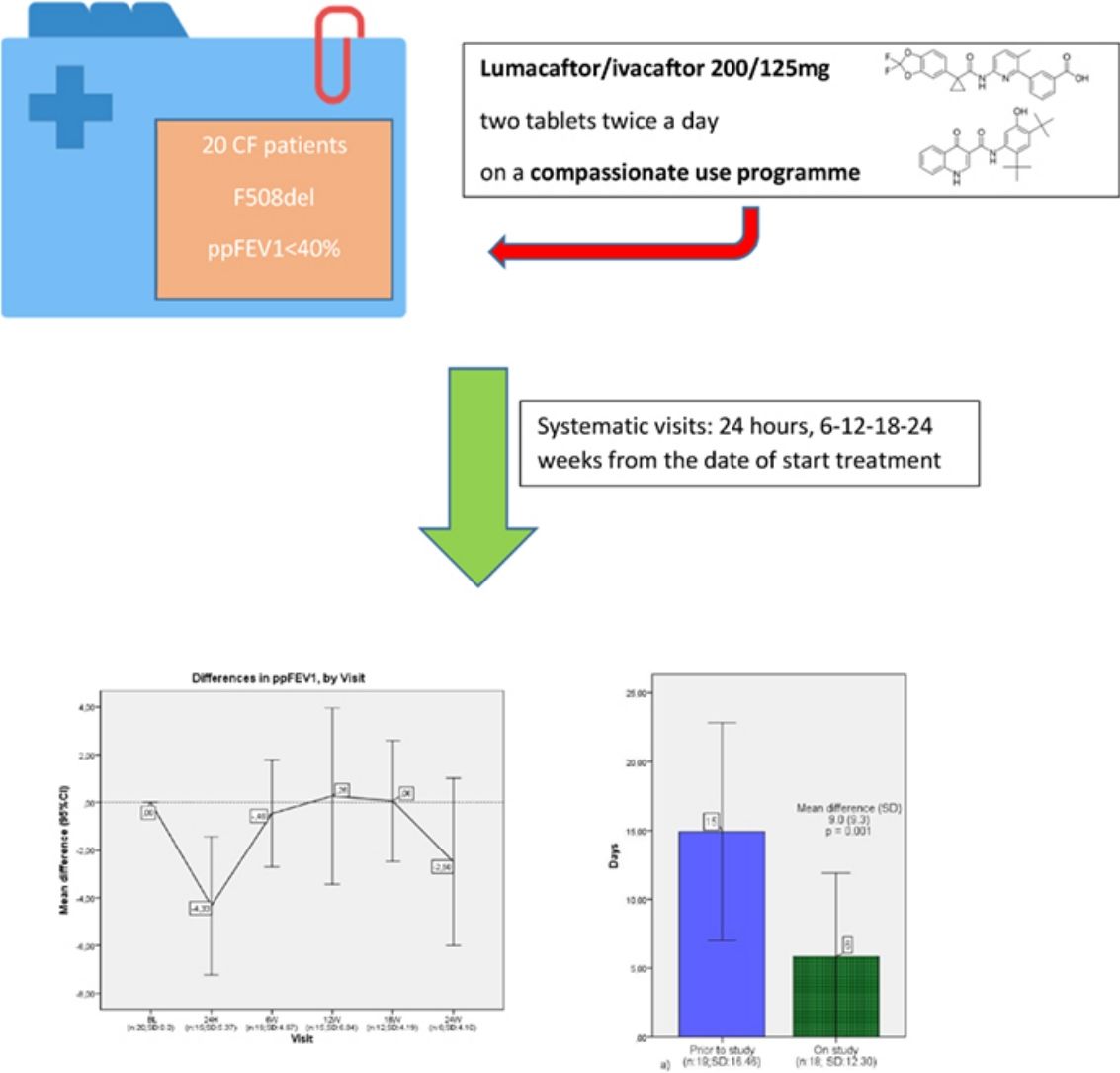
MethodsWe conducted a multicenter, retrospective, observational study to describe the effects of lumacaftor/ivacaftor treatment in real-life in Spain. 20 CF patients were included, all aged 6 and upwards and presented with ppFEV1<40%, chosen from CF units country-wide. For the purposes of the study, they were treated with lumacaftor/ivacaftor 200/125mg two tablets twice a day on a compassionate use programme throughout 2016. The primary endpoint was measured in all of the sample patients. Data were analysed from ppFEV1 at baseline and was measured every 6 months.
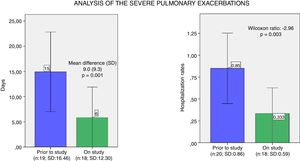
ResultsThe mean age was 26.65 (range of 10–45), while the mean ppFEV1 before the treatment was 32.4% and mean BMI was 19.9kg/m2. We analysed the changes in ppFEV1 and BMI from baseline during the treatment with lumacaftor/ivacaftor, but no differences were found. However, a moderate association between days of intravenous antibiotic needed and the use of lumacaftor/ivacaftor (p=0.001) was established. Indeed, under the lumacaftor/ivacaftor, patients required 5.8 days of intravenous antibiotic treatment compared to 14.9 days prior to study. Also, severe pulmonary exacerbations requiring hospitalisation were statistically fewer under lumacaftor/ivacaftor treatment (p=0.003). Finally, 75% of the sample presented with AEs, which led 35% of the subjects to discontinue the treatment.
ConclusionsWhile treatment with lumacaftor/ivacaftor resulted in an improvement in the number of pulmonary severe exacerbations, no improvement in ppFEV1 or BMI was found.
La mutación causante de fibrosis quística (FQ) más frecuente es la deltaF508 (F508del), presente en el 28% de los pacientes españoles con FQ. Aunque la literatura sobre estudios en vida real en pacientes de FQ homocigotos para F508del tratados con lumacaftor/ivacaftor es escasa, pone de manifiesto la necesidad de contar con mejores estrategias para prevenir los efectos adversos (EA) relacionados con el tratamiento, así como del desarrollo de nuevos fármacos.
MétodosSe realizó un estudio observacional, retrospectivo multicéntrico para describir los efectos del tratamiento con lumacaftor/ivacaftor en vida real en España. Se incluyeron 20 pacientes con FQ, edad superior a los 6 años y ppFEV1<40%, procedentes de unidades de FQ de todo el país. Para los fines del estudio, fueron tratados con 2 comprimidos de lumacaftor/ivacaftor 200/125mg/2 veces al día como parte de un programa de uso compasivo a lo largo de 2016. El criterio de valoración primario se midió en las muestras de todos los pacientes. Los datos de ppFEV1 se analizaron al inicio y cada 6 meses.
ResultadosLa mediana de edad fue de 26,65 (rango: 10-45), mientras que la mediana de ppFEV1 antes del tratamiento fue del 32,4% y la mediana del IMC 19,9kg/m2. No se encontraron diferencias al analizar los cambios de ppVEF1 e IMC desde el inicio y durante el tratamiento con lumacaftor/ivacaftor. Sin embargo, se estableció una asociación moderada entre los días requeridos de antibiótico intravenoso y el uso de lumacaftor/ivacaftor (p=0,001). De hecho, con lumacaftor/ivacaftor, los pacientes requirieron 5,8 días de tratamiento intravenoso con antibiótico, comparado con los 14,9 días previos al estudio. Además, el número de exacerbaciones pulmonares graves que requirieron hospitalización fue estadísticamente menor con lumacaftor/ivacaftor (p=0,003). Por último, el 75% de la muestra presentó EA, lo cual supuso la discontinuación del tratamiento en un 35% de los casos.
ConclusiónEl tratamiento con lumacaftor/ivacaftor mejoró el número de exacerbaciones pulmonares severas, pero no supuso mejora ni en el ppFEV1 ni el IMC.
Cystic fibrosis (CF) is a genetic disease, inherited in an autosomal recessive fashion, which causes the defective function of the cystic fibrosis transmembrane regulator (CFTR) protein. It affects approximately 1 in 5000 newborns in Europe. CF is caused by abnormalities in salt transportation that result from the defective CFTR protein, a chloride channel that regulates the salt content in the fluid that covers cell surfaces in the nose and lungs. Transport of ions such as sodium and chloride generates an electrical potential difference across the airway lining.
The most common mutation that causes CF is F508del, which affects 28% of Spanish CF patients, and causes various defects such as reductions in the folding and trafficking of the CFTR protein to the epithelial cell surface and defective channel gating.
Lumacaftor is a CFTR corrector which selectively increases the processing and trafficking of F508del CFTR to the cell surface. Ivacaftor is a CFTR potentiator which increases the channel opening probability of CFTR on the cell surface, facilitating chloride transport.
TRAFFIC and TRANSPORT were two phase 3 trials, both of which were multi-national, randomised, double-blind, placebo-controlled, parallel group studies in which lumacaftor and ivacaftor were orally administered for 24 weeks.1 Both studies revealed improvements in percent predicted forced expiratory volume in 1s (ppFEV1), nutritional status and pulmonary exacerbation rates. The patients included for both studies were 12 and upwards, with a ppFEV1≥40%. A subsequent efficacy analysis showed decreased values in ppFEV1<40% between screening and baseline in fewer than 10% of the patients. At week 24, improvements in the absolute change in ppFEV1 were observed. Increases in body-mass index (BMI) and a reduction in the number of pulmonary exacerbation events were also observed in both the lumacaftor/ivacaftor dose groups compared with the placebo group. The treatment was generally well tolerated, although the incidence of some respiratory adverse events (AEs) was higher under lumacaftor/ivacaftor than with placebo.2
Between 24 October 2013 and 7 April 2016, 1030 patients from the TRANSPORT and TRAFFIC studies enrolled in PROGRESS, and 1029 subjects subsequently received at least one dose of the drug under study.3 The subjects of the study were treated with lumacaftor 400mg/ivacaftor 250mg every 12h. The most common adverse events observed were infective pulmonary exacerbations, cough, increased sputum and haemoptysis. The results showed a slight improvement in ppFEV1 and BMI in week 96, with a reduction of the annual rate of ppFEV1 decline in lumacaftor/ivacaftor treated patients compared to matched controls.
There has been little research regarding the benefits of lumacaftor/ivacaftor outside of clinical trials and even less data on its safety and effectiveness among patients with ppFEV1 lower than 40%. We therefore sought to obtain data on real-life initiation of lumacaftor/ivacaftor in CF adults with severe lung disease in Spain, where lumacaftor/ivacaftor has been available for compassionate use since February 2016.
The European Medicines Agency (EMA) defines “compassionate use” as a treatment option that allows the use of an unauthorised medicinal product which is under development. In Spain, compassionate use is permitted under the following conditions:
- •
The patient must have a chronically or seriously debilitating disease or one that is considered to be life-threatening, and which cannot be treated satisfactorily with authorised products.
- •
The product must be subject to an application for marketing authorisation, or undergoing clinical trials.
- •
The sponsor of the clinical trials or the applicant for marketing authorisation must have consented to supply the product for compassionate use.
While there are few real-life studies on CF patients who are homozygous F508del treated with lumacaftor/ivacaftor, those that have been undertaken have revealed more respiratory-related AEs and initial declines in lung function compared to the results of clinical trials. Approximately 20–25% patients did not tolerate the treatment due to respiratory AEs,4 which demonstrates the need for better strategies to prevent these effects, as well as the development of newer drugs with better AE profiles. Against this background, our cohort of patients should be closely monitored following initiation of lumacaftor/ivacaftor treatment.
MethodsWe conducted a multicenter, retrospective, observational study to describe the effects of lumacaftor/ivacaftor treatment in real life in Spain.
The inclusion criteria were as follows: CF patients, male or female, aged at least 6 years old at the time of signing informed consent form, homozygous for the F508del-CFTR mutation with ppFEV1 value <40%, adjusted for age, sex, and height at screening and able to understand and comply with the protocol requirements. Beyond this, patients who had experienced a rapid and persistent decline in lung function, defined as at least 20% relative decrease in ppFEV1, could also be included in the study, as well as patients in an active waiting list for lung transplant. Those criteria were the established by the programme MyMAPs for compassionate use of lumacaftor/ivacaftor. In the cases where the patient was younger than 18 years old, the consent form was signed by their parents.
The exclusion criteria were as follows: Patients currently receiving invasive mechanical ventilation, those with abnormal liver function, haemoglobin <10mg/dL or abnormal renal function, history of solid organ or hematologic transplantation, those who were currently pregnant and breast-feeding, and patients who were sexually active with reproductive potential but unwilling to follow contraception requirements.
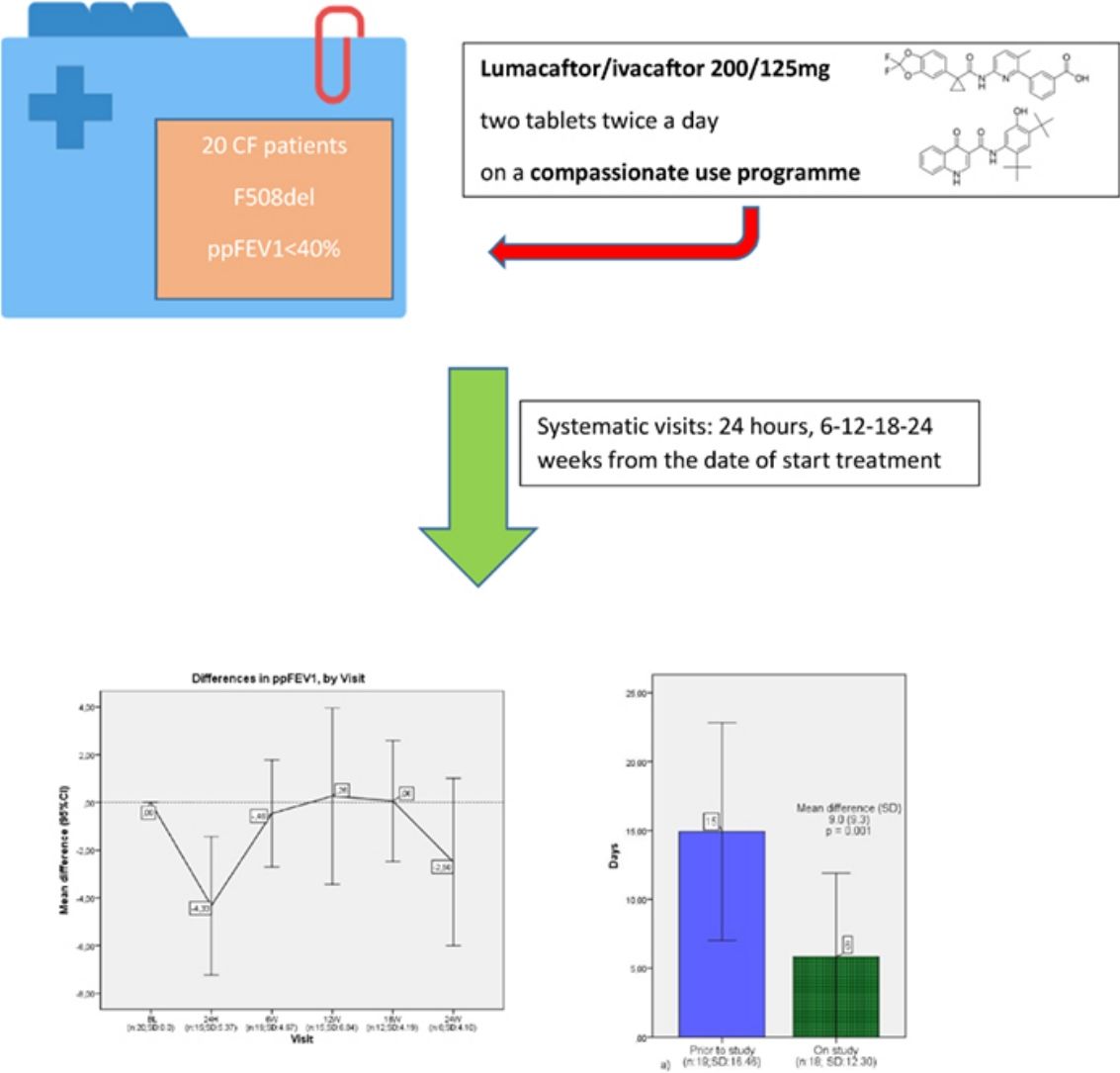
20 CF patients were included from different CF units around the country and treated with lumacaftor/ivacaftor 200/125mg, two tablets twice a day, on compassionate use throughout the year 2016.
These 20 patients were all homozygous F508del, at least 6 years old, with ppFEV1≤40%. All patients were monitored for the first 24h following each administration of a dose, including via spirometry and physiological measures such as oxygen saturation, heart rate, respiratory rate, blood pressure and BMI. Every patient who started lumacaftor/ivacaftor had systematic visits (24h, 6–12–18–24 weeks from the date of start treatment) which included pulmonary function tests. Adverse events data were collected in each visit.
We collected data on number of pulmonary exacerbation from the year prior to the beginning of treatment (distinguishing between mild, moderate and severe) in each visit.
The primary endpoint was measured in all the sampled patients. Data were analysed to ppFEV1 at baseline (<40%) and BMI, was measured for 24 weeks. We considered p values lower than 0.05 as statistically significant. The means of the differences from baseline changes in ppFEV1 and BMI were calculated with a model for repeated measures Greenhouse–Geisser that included study treatment by each visit.
A Shapiro–Wilk test determined a non-normal data distribution. The association between exacerbations in the previous and current year was analysed using the Wilcoxon test. All statistical analyses were performed via the statistical software SPSS, version 19.
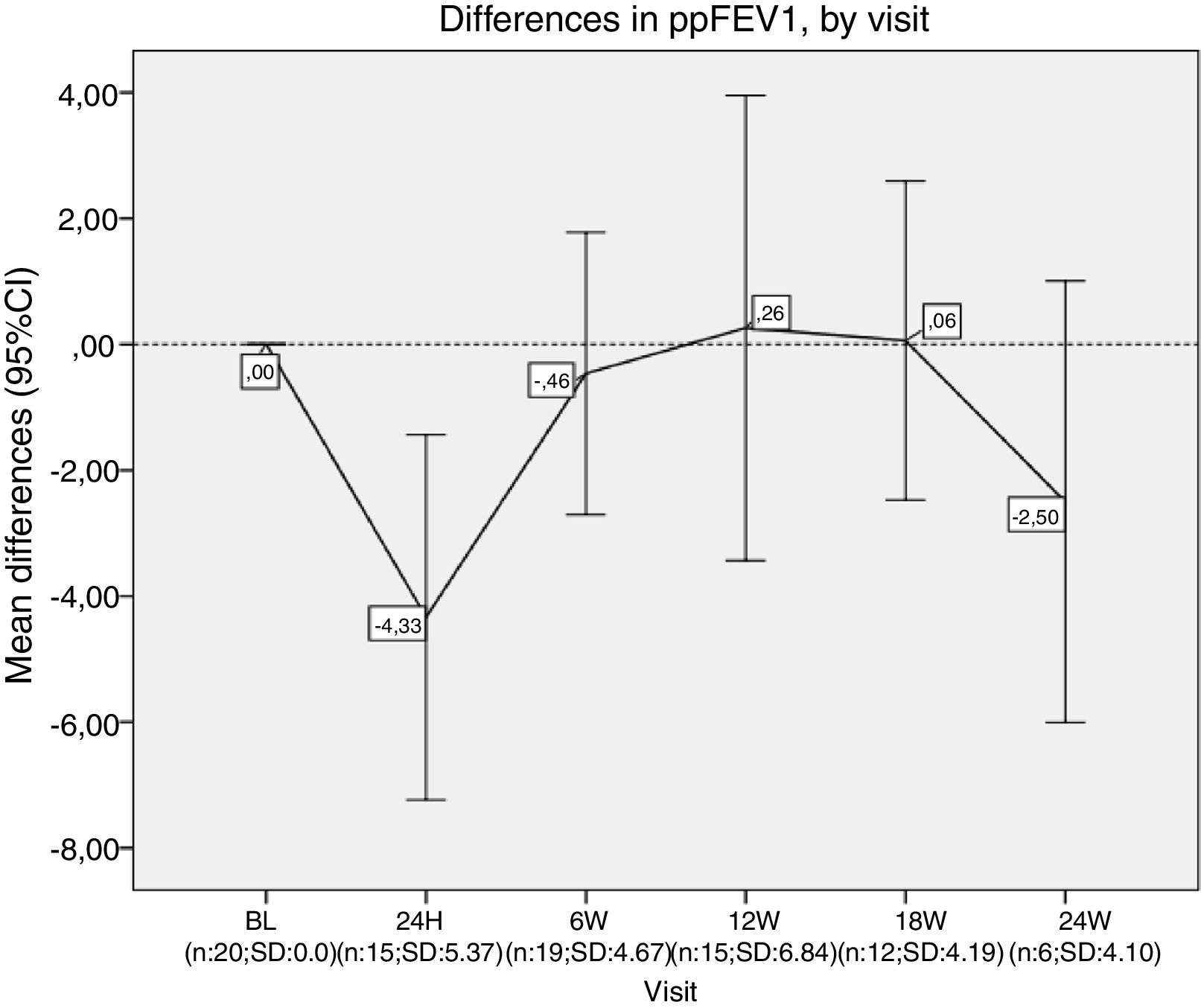
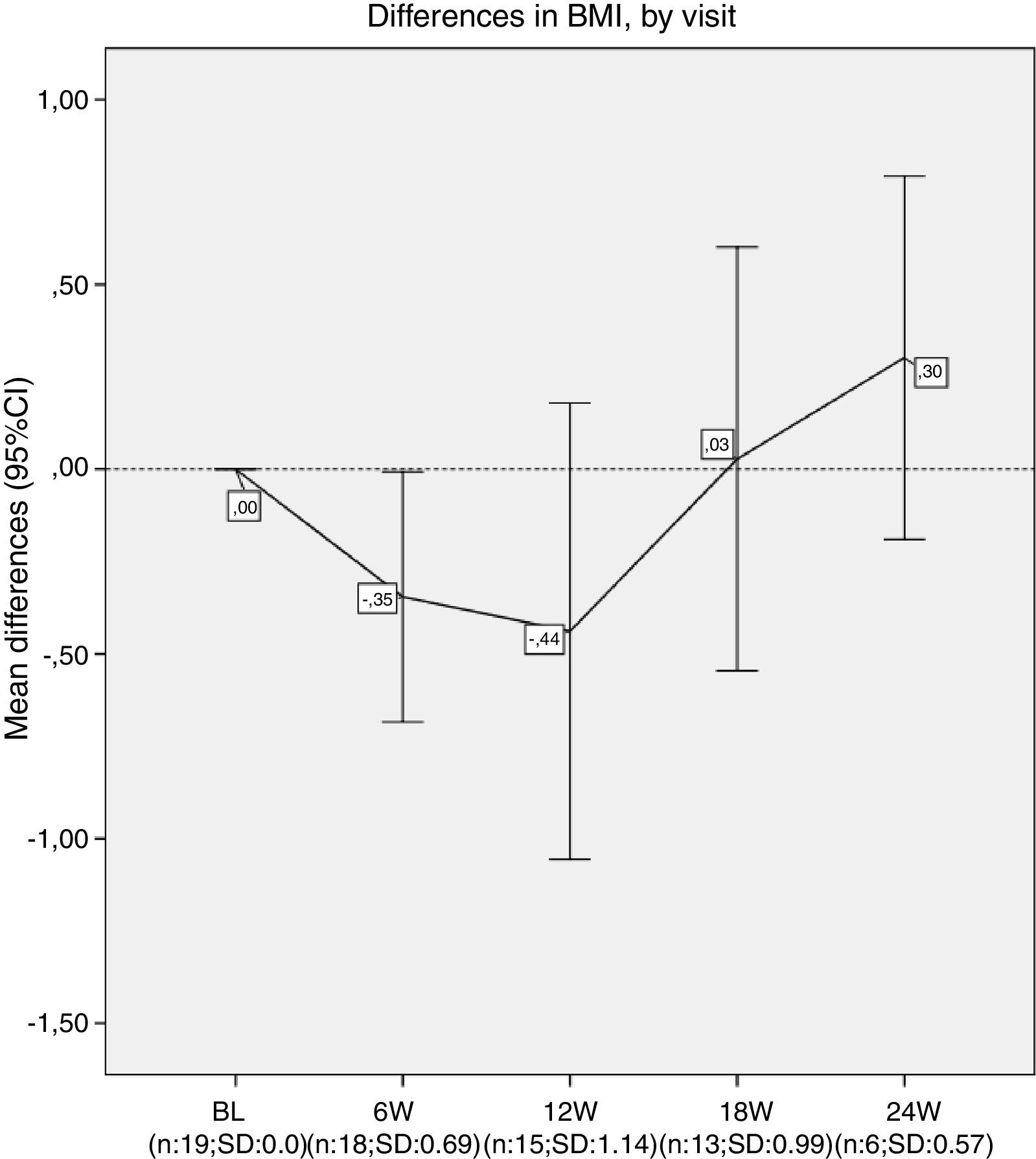
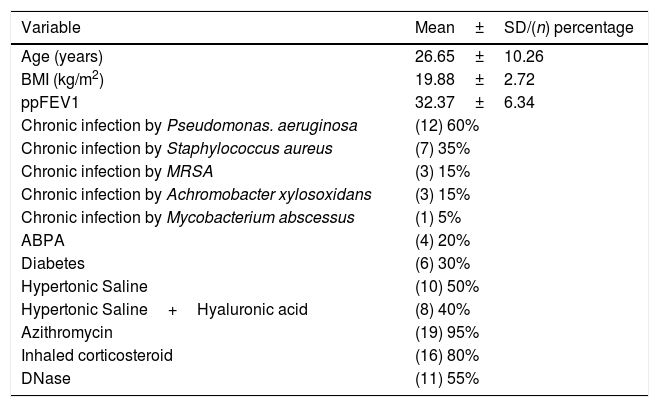
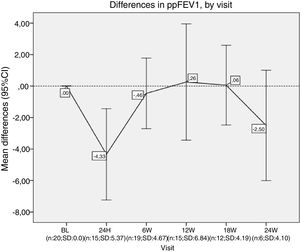
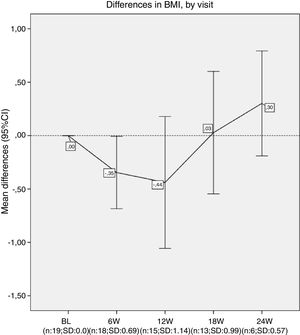
Results20 patients started lumacaftor/ivacaftor 200/125mg treatment. The mean age was 26.65 (range 10–45 years old), the mean ppFEV1 before the treatment was 32.4% and mean BMI 19.9kg/m2. 60% of the cohort was female, 100% had chronic bronchial infection, 60% by Pseudomonas aeruginosa and 35% by Staphylococcus aureus, 30% had diabetes. Almost all the patients used nebulised antibiotics, 55% DNAse, 90% hypertonic saline and 30% oxygen (Table 1). We analysed the absolute changes in ppFEV1 and BMI during the lumacaftor/ivacaftor treatment (figs. 1 and 2).
Demographic features.
| Variable | Mean±SD/(n) percentage |
|---|---|
| Age (years) | 26.65±10.26 |
| BMI (kg/m2) | 19.88±2.72 |
| ppFEV1 | 32.37±6.34 |
| Chronic infection by Pseudomonas. aeruginosa | (12) 60% |
| Chronic infection by Staphylococcus aureus | (7) 35% |
| Chronic infection by MRSA | (3) 15% |
| Chronic infection by Achromobacter xylosoxidans | (3) 15% |
| Chronic infection by Mycobacterium abscessus | (1) 5% |
| ABPA | (4) 20% |
| Diabetes | (6) 30% |
| Hypertonic Saline | (10) 50% |
| Hypertonic Saline+Hyaluronic acid | (8) 40% |
| Azithromycin | (19) 95% |
| Inhaled corticosteroid | (16) 80% |
| DNase | (11) 55% |
MRSA: Methicillin-resistant Staphylococcus aureus.
ABPA: Allergic bronchopulmonary aspergillosis.
ppFEV1: percent predicted forced expiratory volume in one second.
Differences in ppFEV1, by visit. In each visit the mean difference between each visit and the beginning of the treatment (baseline) is represented. ppFEV1: percent predicted forced expiratory volume in one second. CI: confidence interval. N: number of patients. SD: standard deviation. BL: baseline. H: hours. W: week.
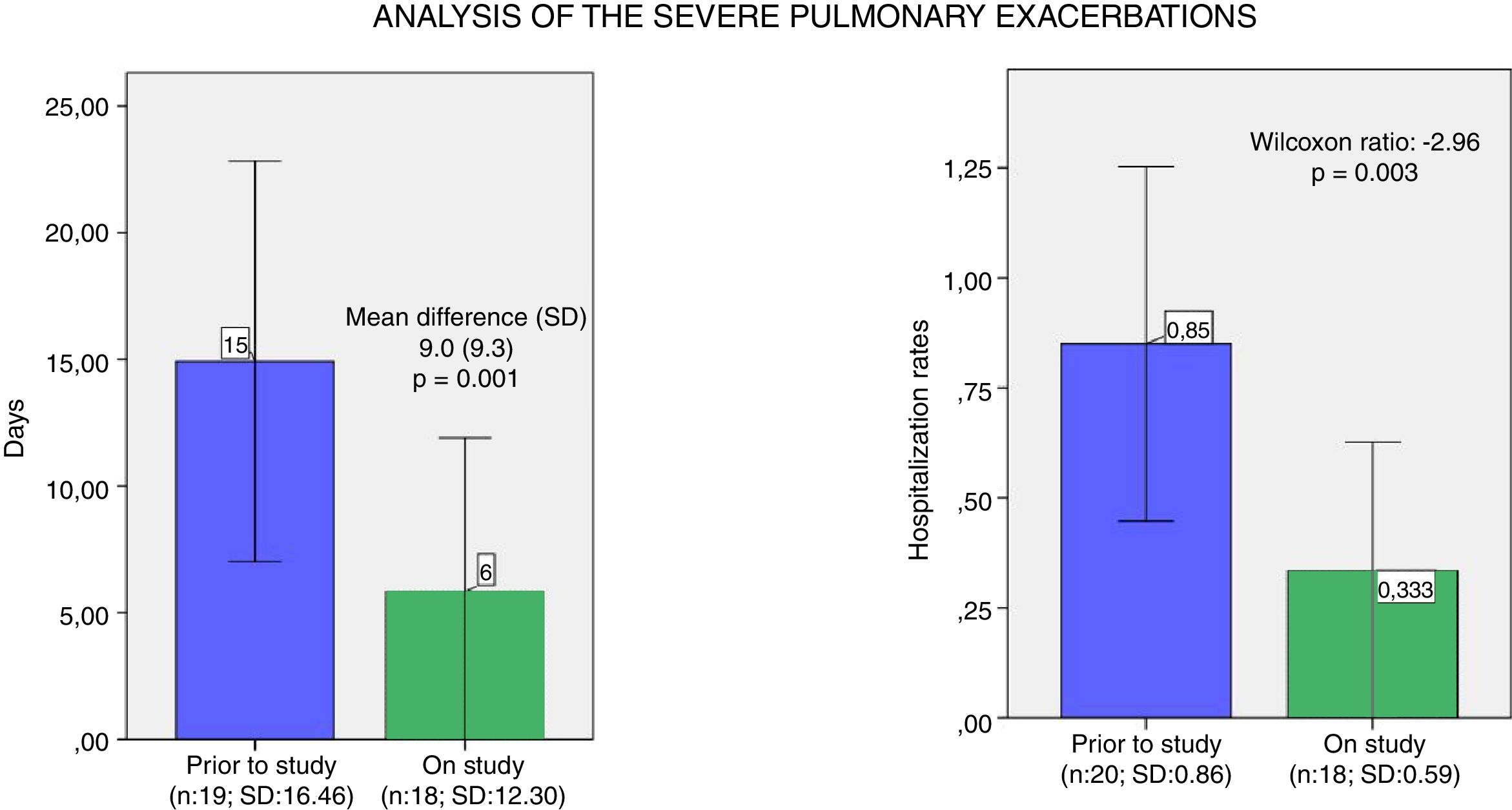
We observed a moderate association between the days of intravenous (i.v.) antibiotic and the use of lumacaftor/ivacaftor treatment, analysed using the Wilcoxon test – which is statistically significant (p=0.001). Also, severe pulmonary exacerbations which required hospitalisation were statistically less common during the year under the lumacaftor/ivacaftor treatment (p=0.003) (Fig. 3).
Analysis of the severe pulmonary exacerbations. (a) Mean and confidence interval of days of intravenous antibiotics one year prior and during the study year. (b) Mean and confidence interval of hospitalisation rates one year prior and during the study year. N: number of patients; SD: standard deviation.
Almost all patients show increase subjective levels of energy and the sensation of less exacerbation.
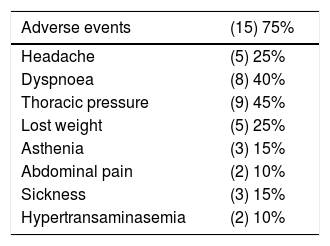
75% of our population presented with AEs, 25% had headaches, 40% dyspnoea, 45% chest tightening and 25% weight loss (Table 2). Consequently, 35% of the subjects discontinued the treatment.
DiscussionThe treatment with lumacaftor/ivacaftor resulted in an improvement of some factors in our CF patients who are F508del homozygous with severe lung diseases, and reported less days of i.v. antibiotics in severe pulmonary exacerbations.
Pulmonary exacerbation (PEx) is a hallmark of cystic fibrosis. The incidence of PEx seems to increase with age.5 In an analysis of data from 11.692 consecutive CF patients included in a large prospective multicentre database (Epidemiologic Study of Cystic Fibrosis),6 the percentage of patients with a PEx during the 6-month observation period increased from 23% for those aged <6 years to 63% for those aged >18 years. Identified risk factors include changes in bacterial density, acquisition of new microorganisms as Pseudomonas aeruginosa, viral infections, impaired lung function, CF related diabetes and other external precipitating.
The main consequences of PEx in patients with CF include higher drug consumption, the need for in-patient care, and patient absenteeism, which results in an increase in the total cost of the disease. Furthermore, PEx has a negative impact on physical and psychosocial health-related quality of life. In addition to the increased morbidity of CF caused by PEx, the annual number of exacerbations has a considerable impact on survival. Therefore, reducing the number of PEx and the total duration of iv antibiotics will reduce the economic cost of the disease. Moreover, the health-related quality of life and surveillance will also improve, which means that reducing severe PEx has very important clinical consequences.
Nevertheless, we did not observe an improvement in lung function after 24 weeks. Our patients presented a decrease of around 5% on ppFEV1 in the first 24h after lumacaftor/ivacaftor administration, and this decline did not recover until week 12 onwards. We had to stop the treatment for one of our patients. Finally, while we observed a slight increase in BMI, this was not statistically significant.
The initial decline in pulmonary function and the increase in respiratory AEs have been described in a study by an Australian research group,7 in which they concluded that lumacaftor/ivacaftor respiratory-related adverse events and declines in lung function are common in patients with ppFEV1<40, meaning that this cohort of patients should be closely monitored following lumacaftor/ivacaftor initiation. Notwithstanding, in a real-life study carried on by a French research group with 53 patients with ppFEV1<40,4 the mean absolute change in ppFEV1 was +2.06% after 1 month of treatment with lumacaftor/ivacaftor (p=0.086), and +3.19% after 3 months (p=0.009); after 1 and 3 months of treatment, about one third of all patients had an absolute change in ppFEV1 of 5% or more in comparison to baseline and 13% had an increase of at least 10% in ppFEV1. Nevertheless, more studies to evaluate the efficacy of lumacaftor/ivacaftor therapy in severe CF lung disease are needed to help us to better-informed future therapeutic decisions.
In our cohort, the patients showed a high percentage of AEs, 25% had headache, 40% dyspnoea, 45% chest tightening and 25% weight loss, and this resulted in the discontinuation of the treatment for 35% of the patients.
As with the study by Dominique Hubert et al.,4 the level of treatment discontinuation was markedly higher than rates in phase 3 clinical trials. This could be explained by the fact that the patients in real-life studies with treatment on compassionate use are not in such stable conditions as in clinical trials. Elborn et al.1 reported that the incidence of dyspnoea in patients with ppFEV1<40% was two times higher than in patients with ppFEV1>40%.
This highlights that the evolution of CF patients taking lumacaftor/ivacaftor with low lung function over the longer term should be further studied.
The median predicted survival for patients with CF is steadily increasing in Europe, and currently life expectancy for cystic fibrosis patients surpasses 40 years. This new class of drugs that is mutation-specific and modulate CFTR function have the potential to be transformational. The editorial written by Schwarz and Harti published in the European Respiratory Journal forecasts that the adult CF population in Europe will increase by 50% by 2025.8 The editorial further highlights that an early and correct diagnosis is paramount and it is our responsibility to improve our knowledge of CF healthcare, and to pass this on to future generations in order to optimise the limited resources for CF care.
Limitations in our study included the retrospective nature of the study, the small sample size and that the selection of patients was based on compassionate use requests for severely ill patients.
Finally, we conclude that our Spanish study in severe CF patients taking lumacaftor/ivacaftor treatment shows an improvement in the number of pulmonary severe exacerbations but no improvement in ppFEV1 and BMI was reported.
Conflict of interestRosa María Girón-Moreno has received honoraria for workshops from Vertex, Teva, Zambon and Esteve.
Esther Quintana-Gallego has received honoraria for workshops and expert comitte from Chiesi, Gilead, Novartis, Praxis y Vertex.
Luis Maiz has received honoraria for workshops from Vertex.
Marta María García-Clemente has received honoraria for workshops from GSK and Chiesi.
Carmen Luna-Paredes has received honoraria for workshops and participate in advisory boards from Vertex.
Pedro Mondejar-López has received honoraria for workshops from Vertex.
Alejandro López-Neyra has received honoraria for workshops from Vertex.
The rest of authors declare no conflict of interests.