Hypersensitivity Pneumonitis (HP) is an immune-mediated disease manifesting as Interstitial Lung Disease (ILD) in susceptible individuals after exposure to an inhaled antigen.1,2 HP is classified as non-fibrotic HP (inflammatory) and fibrotic HP3 and is identified as the third most common ILD4 with high mortality in their fibrotic phenotype. Unfortunately, it can be misdiagnosed as Idiopathic Pulmonary Fibrosis (IPF) or another idiopathic interstitial pneumonia.5 The diagnosis of HP remains complex; there is no gold standard diagnostic test, so the diagnosis is based on the integration of several domains published in two guidelines.1,2 In both, diagnostic criteria are rooted in four domains: (1) exposure identification, (2) high-resolution computed tomography (HRCT), (3) BAL lymphocytosis, and (4) histopathology. In 2021, Alberti et al. proposed an algorithm for diagnosing HP based on the same domains used by both guidelines.6 However, the three algorithms present different approaches and diagnostic certainties for a reliable diagnosis. In this study, we applied the three algorithms to a cohort of patients with HP and other ILDs. The aim of this study was to compare the diagnostic performance of the three algorithms.
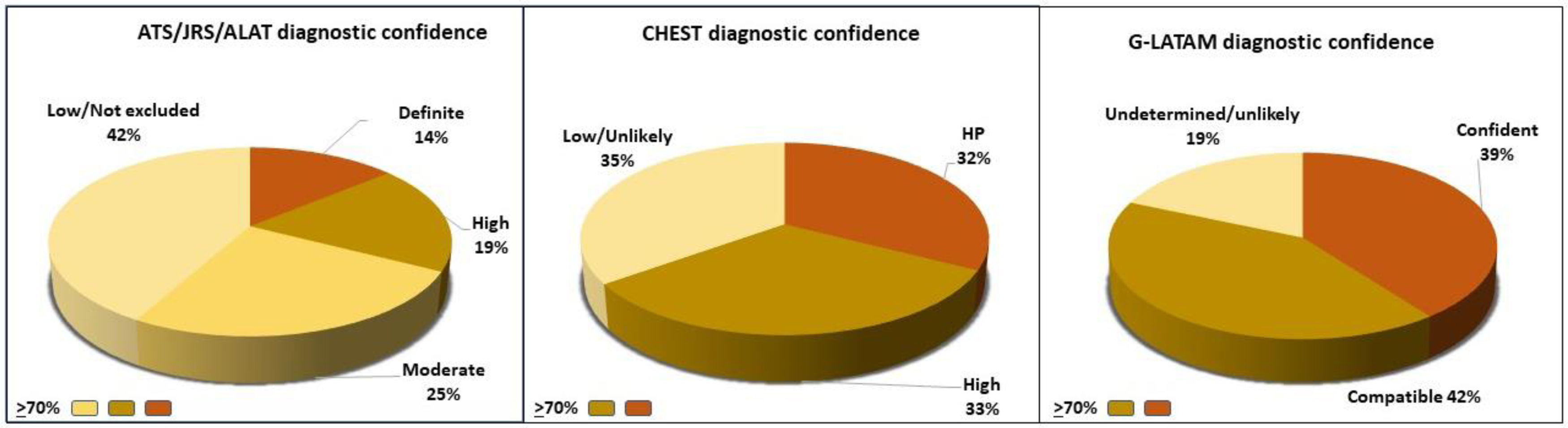
One-hundred patients from our National Institute of Respiratory Diseases were included: 43 with confirmed HP (fibrotic and non-fibrotic) and 57 diagnosed with other ILDs. The diagnosis was established after our Multidisciplinary Discussion Team (MDT) review. Two evaluators were blinded to the final diagnosis and considered a diagnosis of HP with a diagnostic certainty of >70%. This percentage has been previously used for the diagnostic accuracy and validity of an HP diagnostic algorithm in a study that included 31 patients with HP and 50 patients with IPF, and reported that diagnostic confidence >70% had a satisfactory performance for the previous diagnosis of HP by multidisciplinary discussion.7 The ATS/JRS/ALAT guideline presents five grades of confidence: definite (>90% diagnostic likelihood), high confidence (80–89%), moderate confidence (70–79%), low confidence (51–69%), and HP considered not excluded (≤50%).1 The American College of Chest Physicians (CHEST) guideline presents four grades of confidence: confident diagnosis (>90%), provisional high confidence (70–89%), provisional low confidence (51–69%), and unlikely (≤50%).2 Alberti et al. (G-LATAM) present four grades of confidence: Confident (>90%), Compatible (70–89%), Undetermined (50–69%), Unlikely (<50%).6 The primary outcome measure was the diagnostic performance (i.e., specificity and sensitivity) of each algorithm compared to the gold standard, diagnosis determined by MDT. We used mean and standard deviation (SD) for quantitative variables and frequency and percentages to analyze qualitative variables. The Student t-test and Fisher's F exact test were used to compare the different variables of the two groups. This study was approved by the institutional ethics committee (C30-22).
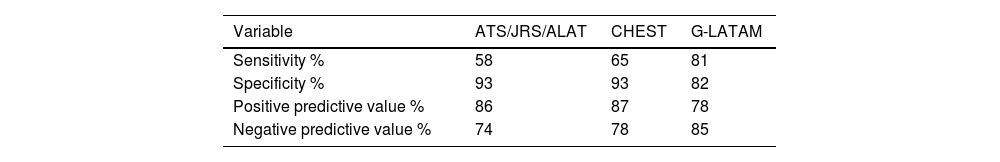
In our cohort of 100 patients, we observed an average age of 58±12 years, and 82% were women. When comparing the HP group and the group with other ILDs, we found differences in age (53±12 vs. 62±11 years, p<0.001) and BAL lymphocytosis (48±21% vs. 21±20%, p<0.001), respectively. Fifty-eight percent of the HP group had an identified antigen, and only 16% had a lung biopsy. Of the 43 patients with HP, 58% were diagnosed with HP with diagnostic confidence of >70% by the ATS/JRS/ALAT algorithm, 65% with CHEST, and 81% with the G-LATAM algorithm (Fig. 1). The diagnostic test analysis revealed that the G-LATAM algorithm had a higher sensitivity (81%) compared to ATS/JRS/ALAT (58%) and CHEST (65%), with a marginal lower positive predictive value, 78% (Table 1).
HP continues to be a diagnostic challenge if we do not follow a consensus definition and recommended criteria.3,8 Hence, the application of algorithms helps improve diagnostic certainty. Three algorithms have been proposed to integrate key domains to diagnose NH; however, although they share the domains, their reading is different.1,2,6 In 2021, our working group compared the performance of the ATS/JRS/ALAT and CHEST guidelines in 144 patients with HP, finding low concordance between the two for definitive/high-confidence diagnosis and indicating that the principal difference between the two guidelines is that the ATS/JRS/ALAT guideline is more restrictive in determining a definitive diagnosis, yielding markedly fewer confident diagnoses.9 Although all domains included in the algorithms are feasible, they are not accessible to all hospital care centers or do not always have conclusive results. For example, although antigen exposure is a fundamental domain, in a large percentage of cases, cannot be identified (30–50%), and specific serum levels of IgG can confirm exposure to the antigen without proving causality with the disease.1 Likewise, as a formal diagnostic criterion, BAL lymphocytosis is relevant for HP diagnosis and integrated into an algorithm. However, the optimal BAL lymphocytosis cut-off still needs to be determined, and different cut-off points has been described: 30%,2,6 40%,7 and 50%,10 which makes it difficult for the pulmonologist to decide which to use, and moreover, not in all the centers this study is performed. HRCT findings are classified as typical, compatible, and indeterminate1,2; however, inflammatory disease may go unrecognized, while fibrotic disease may be misdiagnosed as IPF.11 In patients with suspected HP, but a reliable diagnosis cannot be made based on the available data, a lung biopsy is recommended,12 considering the risk and benefit for the patient. According to the CHEST algorithm, performing a lung biopsy is recommended when additional diagnostic evaluation does not show a reliable diagnosis (low confidence<69%), ATS/JRS/ALAT (high confidence<89%), and G-LATAM (undetermined confidence<69%). Finally, the three algorithms recommend the evaluation by MDT as a final step in decision-making. This study has significant limitations, including being conducted at a single reference center with a relatively small cohort.
Despite its limitations, the G-LATAM algorithm demonstrated better diagnostic performance compared to the obtained with ATS/JRS/ALAT and CHEST based on the domains integrated into the algorithms. However, validating these findings in other larger HP cohorts is crucial.
Contribution of Each AuthorThe authors confirm contribution to the paper as follows: study conception and design: Buendia-Roldan I and Aguilar-Duran H; data collection: Aguilar-Duran H; analysis and interpretation of results: Aguilar-Duran H, Buendia-Roldan I, Selman-M; draft manuscript preparation: Aguilar-Duran H and Buendia-Roldan I, Selman-M. All authors reviewed the results and approved the final version of the manuscript.
List of ResearchersNot applicable.
Artificial Intelligence InvolvementNo artificial intelligence was used.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestsThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
Not applicable.