The aim of this study was to analyze the clinical and genetic characteristics of young lung cancer cases, and to compare them with those of older cases.
MethodsWe used the Thoracic Tumors Registry (TTR) as a data source representative of lung cancer cases diagnosed in Spain, and included all cases registered until 9/01/2023 which had information on age at diagnosis or the data needed to calculate it. We performed a descriptive statistical analysis and fitted logistic regressions to analyze how different characteristics influenced being a younger lung cancer patient.
ResultsA total of 26,336 subjects were included. Lung cancer cases <50 years old had a higher probability of being women (OR: 1.38; 95% CI: 1.21–1.57), being in stage III or IV (OR: 1.32; 95% CI: 1.08–1.62), not having comorbidities (OR: 5.21; 95% CI: 4.59–5.91), presenting with symptoms at diagnosis (OR: 1.53; 95% CI: 1.29–1.81), and having ALK translocation (OR: 7.61; 95% CI: 1.25–46.32) and HER2 mutation (OR: 5.71; 95% CI: 1.34–24.33), compared with subjects ≥50 years. Among subjects <35 years old (n=61), our study observed a higher proportion of women (59.0% vs. 26.6%; p<0.001), never smokers (45.8% vs. 10.3%; p<0.001), no comorbidities (21.3% vs. 74.0%; p<0.001); ALK translocation (33.3% vs. 4.4%; p<0.001) and ROS1 mutation (14.3% vs. 2.3%; p=0.01), compared with subjects ≥35 years.
ConclusionsLung cancer displays differences by age at diagnosis which may have important implications for its clinical management.
In 2020, lung cancer ranked as the principal cause of cancer-related mortality with 18% of total deaths worldwide, followed by colorectal cancer and liver cancer, with 9.4% and 8.3% of all cancer-related deaths, respectively.1 Age is an important factor in the appearance and progression of lung cancer. Hence, according to data sourced from the Survival Epidemiology and End Results (SEER), the median age at diagnosis stands at 71 years,2 though there is a wide age distribution. The lung cancer mortality rate in subjects aged 50 years and older was 84.6 deaths per 100,000 population in 2020, whereas for subjects aged under 50 years it was 1.2 per 100,000 population.2 This indicates that the incidence of lung cancer in persons under the age of 50 years is not that uncommon.1 In Spain, an estimated 22,266 lung cancer cases are going to be diagnosed in 2023, 7,146 of whom can be assumed to be subjects under the age of 65 years, accounting for 32% of all lung cancer cases.3
In recent years, a number of studies have suggested that there are differences in the clinical characteristics of subjects who develop lung cancer at earlier ages, as compared with older subjects,4–13 describing lung cancer in young subjects as a unique entity. Previous studies have concluded that lung cancer which develop in younger subjects tend to appear more frequently in women and never smokers.14,15 Furthermore, in younger subjects, there is a predominance of adenocarcinoma and tumors at more advanced stages.6,7,10 Moreover, a number of studies have observed a greater proportion of certain genotypes or genetic alterations in younger subjects, including a higher frequency of mutations of the epidermal growth factor receptor (EGFR) and translocations involving anaplastic lymphoma kinase (ALK).16,17 Yet, the results differ between genotypes,18 and a recent systematic review has concluded that there are discrepancies in the association between the presence of genetic alterations and age.12 The presence of some of these alterations is associated with better survival.7,19 While different studies have observed better prognosis in younger subjects,13,17 other studies however report lower survival in comparison with older subjects.10,11,15
The differences observed suggest that the biology of lung cancer may differ by age at diagnosis,13 something that can result in a more aggressive disease in young subjects, as indeed happens with other tumors diagnosed at early ages.20 Identification of possible differences, both clinical and genetic, between younger and older lung cancer cases may have important implications when it comes to the diagnosis, treatment and clinical care of patients, furnishing information on the factors that influence the development of the disease in younger subjects and leading to the potential detection of driver genes in these subjects, which may in turn have implications for the treatment strategy to be pursued.10
Due to the low incidence of lung cancer in young population, there are hardly any studies with a sufficient sample size that have compared the clinical and genetic characteristics of lung cancer by age at diagnosis, particularly in subjects aged under 50 years or in the Caucasian population. This study therefore sought to analyze the clinical and genetic characteristics of lung cancer cases diagnosed in Spain, by age at diagnosis, and compare these characteristics in subjects under the age of 50 with those in subjects aged 50 years and older. To achieve this, we used a representative database of lung cancer cases diagnosed in Spain.
MethodsStudy Design and Sample SelectionWe carried out an analytical cross-sectional study, using the Thoracic Tumors Registry (TTR) of the Spanish Lung Cancer Group (Grupo Español de Cáncer de Pulmón) as our data source. The TTR is a monographic lung cancer registry whose methodology has been previously described.21 In summary, the TTR is a multicenter prospective study involving 80 hospitals throughout Spain, designed with a consecutive sampling method. The cases are subjects diagnosed with lung cancer or other thoracic tumors, without any restriction on gender or age, and regardless of whether they are receiving treatment or not. The cases are collected by oncologists at the participating hospitals. This registry has recently been shown to be representative of lung cancer cases diagnosed in Spain by sex and age.21 The TTR was registered in ClinicalTrials.gov (NCT02942458), and the study protocol was approved by the institutional committee of the Puerta de Hierro University Teaching Hospital (Majadahonda, Madrid) (no. PI 148/15).
For study purposes, we used all cancer cases included in the TTR until 9 January 2023 which had information on age at diagnosis or the data needed to calculate it (date of birth and date at diagnosis). Subjects diagnosed with thymoma or mesothelioma (n=360) were excluded from the analysis.
For each subject included, the following characteristics were collected: sex, age, smoking habit, tobacco use (in pack-years), stage at diagnosis, histologic type, presence of comorbidities, and presence of symptoms at diagnosis. Age was categorized into: under 50 years (with a subgroup of cases aged 35 years or younger at diagnosis); and 50 years and older. In addition, we recorded the genetic alterations of subjects who underwent a series of molecular determinations at diagnosis, including the following: EGFR, ALK, KRAS, BRAF, mutation in HER2, ROS1, NTRK, FGFR1, PDL1, HER2, RET, and MET.
Statistical AnalysisWe first described the clinical characteristics of subjects diagnosed with lung cancer included in the study, both overall and by age at diagnosis, categorized as under 50 years and 50 years and older. Quantitative variables were expressed as median and interquartile range, and categorical variables as absolute and relative frequencies.
To describe the genetic alterations of the sample, percent positivity was calculated. For each molecular determination analyzed, the number of subjects with a positive determination (i.e., alteration) was divided by the number of subjects in whom this molecular determination had been measured, by age group.
For each characteristic, differences between groups were evaluated using bivariate logistic regression. Variables with a p<0.20 in the bivariate analysis were included in two multivariate logistic regression models: clinical characteristics were included in one and genetic alterations in the other, with both being adjusted for the variables included in the model and for sex and smoking habit. We calculated the odds ratios (ORs) accompanied by their 95% confidence intervals (95% CIs).
We also performed an additional analysis, by dividing the sample into subjects aged under 35 years and aged 35 years and older and carrying out a bivariate analysis. The small sample size in the group of subjects aged under 35 years meant that a multivariate logistic regression could not be performed.
Statistical significance was set at p<0.05. All statistical analyses were performed using the Stata v.17. computer software program.
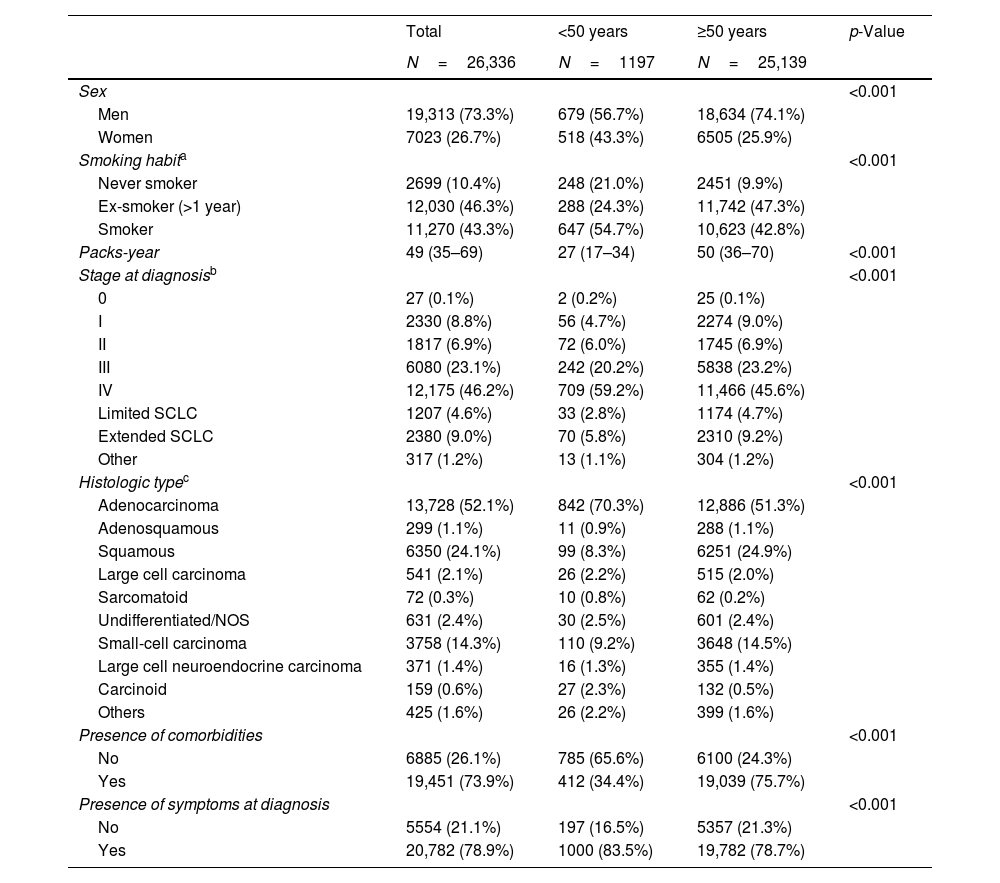
ResultsA total of 27,416 lung cancer cases were included in the TTR at 9 January 2023. Ultimately, 26,336 subjects who fulfilled the preestablished selection criteria were included in this analysis. Tables 1 and 2 show the clinical and genetic characteristics of the sample, both overall and by age group at diagnosis.
Clinical Characteristics of Subjects, Both Overall and by Age Group at Diagnosis.
| Total | <50 years | ≥50 years | p-Value | |
|---|---|---|---|---|
| N=26,336 | N=1197 | N=25,139 | ||
| Sex | <0.001 | |||
| Men | 19,313 (73.3%) | 679 (56.7%) | 18,634 (74.1%) | |
| Women | 7023 (26.7%) | 518 (43.3%) | 6505 (25.9%) | |
| Smoking habita | <0.001 | |||
| Never smoker | 2699 (10.4%) | 248 (21.0%) | 2451 (9.9%) | |
| Ex-smoker (>1 year) | 12,030 (46.3%) | 288 (24.3%) | 11,742 (47.3%) | |
| Smoker | 11,270 (43.3%) | 647 (54.7%) | 10,623 (42.8%) | |
| Packs-year | 49 (35–69) | 27 (17–34) | 50 (36–70) | <0.001 |
| Stage at diagnosisb | <0.001 | |||
| 0 | 27 (0.1%) | 2 (0.2%) | 25 (0.1%) | |
| I | 2330 (8.8%) | 56 (4.7%) | 2274 (9.0%) | |
| II | 1817 (6.9%) | 72 (6.0%) | 1745 (6.9%) | |
| III | 6080 (23.1%) | 242 (20.2%) | 5838 (23.2%) | |
| IV | 12,175 (46.2%) | 709 (59.2%) | 11,466 (45.6%) | |
| Limited SCLC | 1207 (4.6%) | 33 (2.8%) | 1174 (4.7%) | |
| Extended SCLC | 2380 (9.0%) | 70 (5.8%) | 2310 (9.2%) | |
| Other | 317 (1.2%) | 13 (1.1%) | 304 (1.2%) | |
| Histologic typec | <0.001 | |||
| Adenocarcinoma | 13,728 (52.1%) | 842 (70.3%) | 12,886 (51.3%) | |
| Adenosquamous | 299 (1.1%) | 11 (0.9%) | 288 (1.1%) | |
| Squamous | 6350 (24.1%) | 99 (8.3%) | 6251 (24.9%) | |
| Large cell carcinoma | 541 (2.1%) | 26 (2.2%) | 515 (2.0%) | |
| Sarcomatoid | 72 (0.3%) | 10 (0.8%) | 62 (0.2%) | |
| Undifferentiated/NOS | 631 (2.4%) | 30 (2.5%) | 601 (2.4%) | |
| Small-cell carcinoma | 3758 (14.3%) | 110 (9.2%) | 3648 (14.5%) | |
| Large cell neuroendocrine carcinoma | 371 (1.4%) | 16 (1.3%) | 355 (1.4%) | |
| Carcinoid | 159 (0.6%) | 27 (2.3%) | 132 (0.5%) | |
| Others | 425 (1.6%) | 26 (2.2%) | 399 (1.6%) | |
| Presence of comorbidities | <0.001 | |||
| No | 6885 (26.1%) | 785 (65.6%) | 6100 (24.3%) | |
| Yes | 19,451 (73.9%) | 412 (34.4%) | 19,039 (75.7%) | |
| Presence of symptoms at diagnosis | <0.001 | |||
| No | 5554 (21.1%) | 197 (16.5%) | 5357 (21.3%) | |
| Yes | 20,782 (78.9%) | 1000 (83.5%) | 19,782 (78.7%) |
IQR: interquartile range; NOS: not otherwise specified; SCLC: small-cell lung cancer.
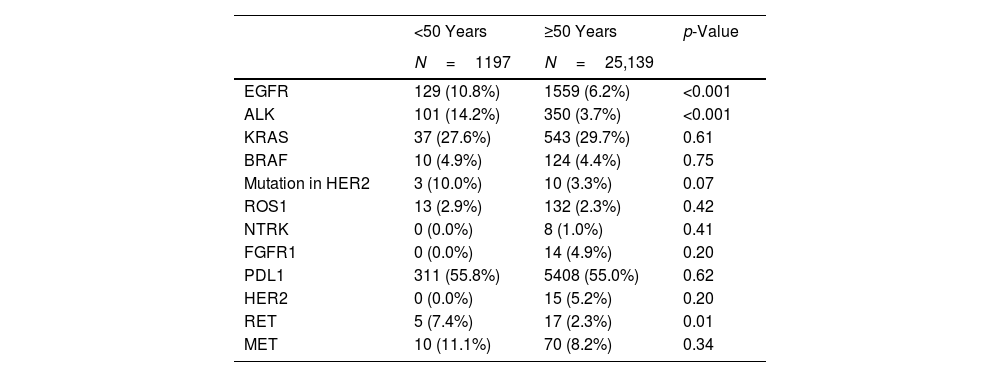
Number of Subjects With Positive Molecular Determinations (Alterations) and Percent Positivity,a by Age Group at Diagnosis.
| <50 Years | ≥50 Years | p-Value | |
|---|---|---|---|
| N=1197 | N=25,139 | ||
| EGFR | 129 (10.8%) | 1559 (6.2%) | <0.001 |
| ALK | 101 (14.2%) | 350 (3.7%) | <0.001 |
| KRAS | 37 (27.6%) | 543 (29.7%) | 0.61 |
| BRAF | 10 (4.9%) | 124 (4.4%) | 0.75 |
| Mutation in HER2 | 3 (10.0%) | 10 (3.3%) | 0.07 |
| ROS1 | 13 (2.9%) | 132 (2.3%) | 0.42 |
| NTRK | 0 (0.0%) | 8 (1.0%) | 0.41 |
| FGFR1 | 0 (0.0%) | 14 (4.9%) | 0.20 |
| PDL1 | 311 (55.8%) | 5408 (55.0%) | 0.62 |
| HER2 | 0 (0.0%) | 15 (5.2%) | 0.20 |
| RET | 5 (7.4%) | 17 (2.3%) | 0.01 |
| MET | 10 (11.1%) | 70 (8.2%) | 0.34 |
The proportion of women was higher in subjects aged under 50 years (43.3% vs. 25.9%; p<0.001), as were the proportions of never smokers (21.0% vs. 9.9%, p<0.001) and of subjects without comorbidity (65.6% vs. 24.3%; p<0.001), as compared with subjects aged 50 years and older. Similarly, intensity of smoking in pack-years was lower in subjects under 50 years of age (27 vs. 50; p<0.001) (Table 1). Higher proportions of EGFR, ALK and RET alterations were observed in subjects under the age of 50 years (p<0.05) than in subjects aged 50 years and older (Table 2).
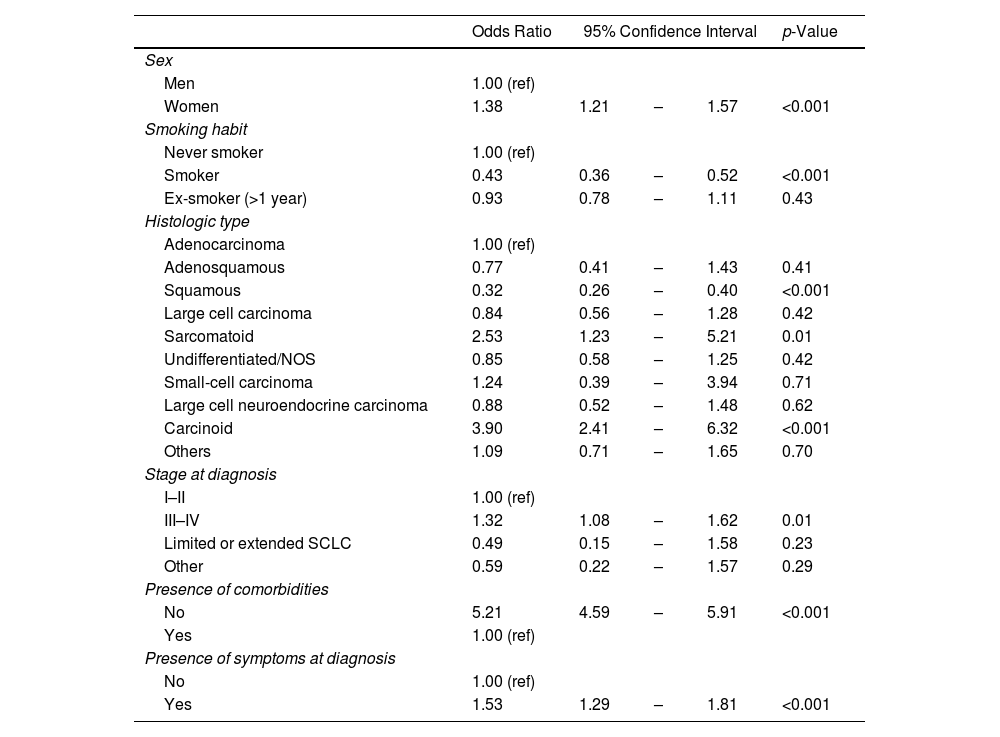
The multivariate analysis of the clinical characteristics of subjects (Table 3) showed that lung cancer cases aged under 50 years had a higher likelihood of being women (OR 1.38; 95% CI 1.21–1.57), presenting with stages III or IV at diagnosis (OR 1.32; 95% CI 1.08–1.62), not having comorbidities (OR 5.21; 95% CI 4.59–5.91), and presenting with symptoms at diagnosis (OR 1.53; 95% CI 1.29–1.81) than did subjects aged 50 years and older.
Multivariate Logistic Regression of the Clinical Characteristics of Subjects: Subjects Under the Age of 50 Years Compared With Subjects Aged 50 Years and Older.
| Odds Ratio | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| Sex | |||||
| Men | 1.00 (ref) | ||||
| Women | 1.38 | 1.21 | – | 1.57 | <0.001 |
| Smoking habit | |||||
| Never smoker | 1.00 (ref) | ||||
| Smoker | 0.43 | 0.36 | – | 0.52 | <0.001 |
| Ex-smoker (>1 year) | 0.93 | 0.78 | – | 1.11 | 0.43 |
| Histologic type | |||||
| Adenocarcinoma | 1.00 (ref) | ||||
| Adenosquamous | 0.77 | 0.41 | – | 1.43 | 0.41 |
| Squamous | 0.32 | 0.26 | – | 0.40 | <0.001 |
| Large cell carcinoma | 0.84 | 0.56 | – | 1.28 | 0.42 |
| Sarcomatoid | 2.53 | 1.23 | – | 5.21 | 0.01 |
| Undifferentiated/NOS | 0.85 | 0.58 | – | 1.25 | 0.42 |
| Small-cell carcinoma | 1.24 | 0.39 | – | 3.94 | 0.71 |
| Large cell neuroendocrine carcinoma | 0.88 | 0.52 | – | 1.48 | 0.62 |
| Carcinoid | 3.90 | 2.41 | – | 6.32 | <0.001 |
| Others | 1.09 | 0.71 | – | 1.65 | 0.70 |
| Stage at diagnosis | |||||
| I–II | 1.00 (ref) | ||||
| III–IV | 1.32 | 1.08 | – | 1.62 | 0.01 |
| Limited or extended SCLC | 0.49 | 0.15 | – | 1.58 | 0.23 |
| Other | 0.59 | 0.22 | – | 1.57 | 0.29 |
| Presence of comorbidities | |||||
| No | 5.21 | 4.59 | – | 5.91 | <0.001 |
| Yes | 1.00 (ref) | ||||
| Presence of symptoms at diagnosis | |||||
| No | 1.00 (ref) | ||||
| Yes | 1.53 | 1.29 | – | 1.81 | <0.001 |
n=25,995. A total of 341 participants had missing data for at least one variable included in the model: 337 were missing data for smoking habit, 2 for histological type, and 3 for stage at diagnosis (one participant had missing information for both histological type and stage at diagnosis).
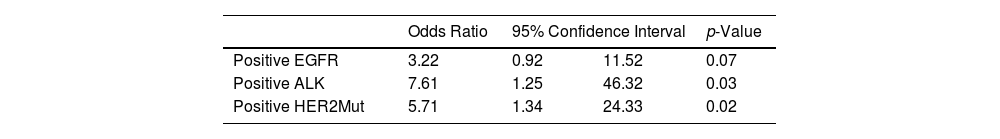
Table 4 shows the logistic regression of genetic alterations, adjusted for sex and smoking habit. Subjects under the age of 50 years had a higher risk of presenting with ALK translocation (OR 7.61; 95% CI 1.25–46.32) and mutation in HER2 (OR 5.71; 95% CI 1.34–24.33) than did subjects aged 50 years and older.
Multivariate Logistic Regression of Genetic Characteristicsa: Subjects Under the Age of 50 Years Compared With Subjects Aged 50 Years and Older.
| Odds Ratio | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Positive EGFR | 3.22 | 0.92 | 11.52 | 0.07 |
| Positive ALK | 7.61 | 1.25 | 46.32 | 0.03 |
| Positive HER2Mut | 5.71 | 1.34 | 24.33 | 0.02 |
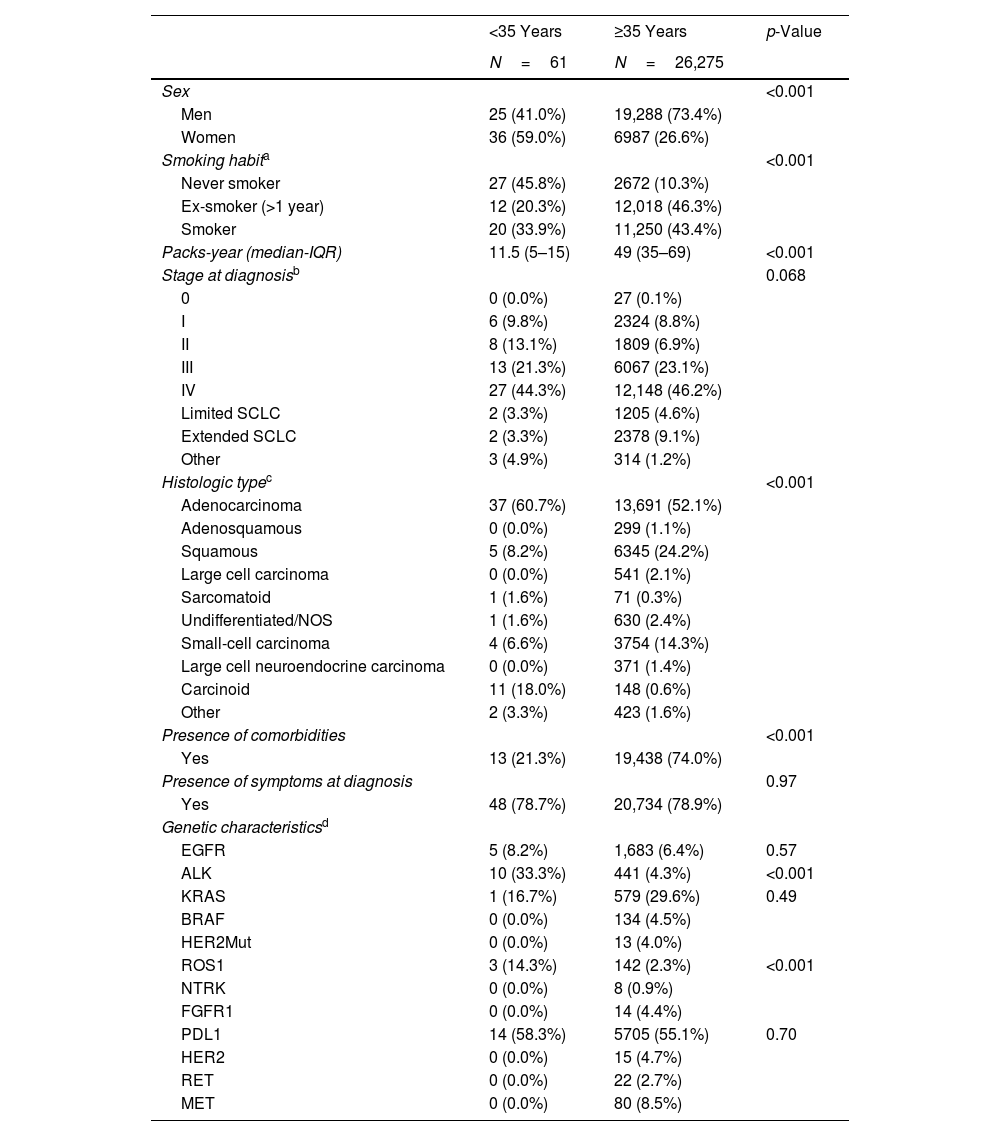
Among subjects under the age of 35 years (n=61), there was a higher proportion of women (59.0% vs. 26.6%; p<0.001), never smokers (45.8% vs. 10.3%; p<0.001), subjects without comorbidities (21.3% vs. 78.9%; p<0.001), and with ALK translocation (33.3% vs. 4.3%; p<0.001) and mutation in ROS1 (14.3% vs. 2.3%; p=0.01), as compared with subjects aged 35 years and older (Table 5).
Description of the Sample by Age at Diagnosis: Subjects Under the Age of 35 Years Compared With Subjects Aged 35 Years and Older.
| <35 Years | ≥35 Years | p-Value | |
|---|---|---|---|
| N=61 | N=26,275 | ||
| Sex | <0.001 | ||
| Men | 25 (41.0%) | 19,288 (73.4%) | |
| Women | 36 (59.0%) | 6987 (26.6%) | |
| Smoking habita | <0.001 | ||
| Never smoker | 27 (45.8%) | 2672 (10.3%) | |
| Ex-smoker (>1 year) | 12 (20.3%) | 12,018 (46.3%) | |
| Smoker | 20 (33.9%) | 11,250 (43.4%) | |
| Packs-year (median-IQR) | 11.5 (5–15) | 49 (35–69) | <0.001 |
| Stage at diagnosisb | 0.068 | ||
| 0 | 0 (0.0%) | 27 (0.1%) | |
| I | 6 (9.8%) | 2324 (8.8%) | |
| II | 8 (13.1%) | 1809 (6.9%) | |
| III | 13 (21.3%) | 6067 (23.1%) | |
| IV | 27 (44.3%) | 12,148 (46.2%) | |
| Limited SCLC | 2 (3.3%) | 1205 (4.6%) | |
| Extended SCLC | 2 (3.3%) | 2378 (9.1%) | |
| Other | 3 (4.9%) | 314 (1.2%) | |
| Histologic typec | <0.001 | ||
| Adenocarcinoma | 37 (60.7%) | 13,691 (52.1%) | |
| Adenosquamous | 0 (0.0%) | 299 (1.1%) | |
| Squamous | 5 (8.2%) | 6345 (24.2%) | |
| Large cell carcinoma | 0 (0.0%) | 541 (2.1%) | |
| Sarcomatoid | 1 (1.6%) | 71 (0.3%) | |
| Undifferentiated/NOS | 1 (1.6%) | 630 (2.4%) | |
| Small-cell carcinoma | 4 (6.6%) | 3754 (14.3%) | |
| Large cell neuroendocrine carcinoma | 0 (0.0%) | 371 (1.4%) | |
| Carcinoid | 11 (18.0%) | 148 (0.6%) | |
| Other | 2 (3.3%) | 423 (1.6%) | |
| Presence of comorbidities | <0.001 | ||
| Yes | 13 (21.3%) | 19,438 (74.0%) | |
| Presence of symptoms at diagnosis | 0.97 | ||
| Yes | 48 (78.7%) | 20,734 (78.9%) | |
| Genetic characteristicsd | |||
| EGFR | 5 (8.2%) | 1,683 (6.4%) | 0.57 |
| ALK | 10 (33.3%) | 441 (4.3%) | <0.001 |
| KRAS | 1 (16.7%) | 579 (29.6%) | 0.49 |
| BRAF | 0 (0.0%) | 134 (4.5%) | |
| HER2Mut | 0 (0.0%) | 13 (4.0%) | |
| ROS1 | 3 (14.3%) | 142 (2.3%) | <0.001 |
| NTRK | 0 (0.0%) | 8 (0.9%) | |
| FGFR1 | 0 (0.0%) | 14 (4.4%) | |
| PDL1 | 14 (58.3%) | 5705 (55.1%) | 0.70 |
| HER2 | 0 (0.0%) | 15 (4.7%) | |
| RET | 0 (0.0%) | 22 (2.7%) | |
| MET | 0 (0.0%) | 80 (8.5%) | |
The table shows the number of subjects who tested positive on each determination and percent positivity. Percent positivity was calculated by dividing the number of subjects with positive determination by the total number of subjects in whom this determination had been measured, for each of the age groups.
The results of this study show that lung cancer cases diagnosed before the age of 50 years (as well as 35 years) present with clinical and genetic characteristics different from those of subjects diagnosed at a higher age. Diagnosis in younger subjects is thus more frequent in women, with a greater predominance of never smokers, a more advanced stage, and a higher frequency of adenocarcinoma. In terms of genetic alterations, subjects under the age of 50 years at diagnosis are seven times more likely to present with ALK translocation and 5 times more likely to have mutations in HER2.
The results obtained suggest that, in general, diagnosis of lung cancer before the age of 50 and 35 years occurs more frequently in women. However, due to the low sample size of subjects under 35 years of age, future studies should confirm these findings. Additionally, diagnosis before the age of 50 years occur at a later stage (presence of stage IV at diagnosis is 59% in younger subjects vs. 45.6% in older subjects). Other studies have also observed a later diagnosis of lung cancer in younger subjects. Subramanian et al., 14 identified 57.4% of cases in stage IV among subjects aged 40 years and younger vs. 43.0% of cases of advanced cancer in older subjects; and the results of Rich et al.,8 are similar. There could be a number of reasons for this late diagnosis: one of these is that physicians might wait longer before performing imaging tests, as a result of ruling out the possibility of lung cancer in younger persons. It could also be attributed to the fact that the youngest subjects appear to delay longer in presenting with symptoms suggestive of lung cancer, and therefore take longer to consult their physician.6,15 It should be noted, however, that, unlike other studies,7 this difference in stage at diagnosis was not observed when subjects aged under 35 years were analyzed separately. Another possible explanation is that pulmonary cancers in young subjects are more aggressive. This is a plausible hypothesis, since doubling time would be more rapid on patients presenting at a younger age and thus increase the likelihood of their being diagnosed at an advanced stage.
Another important result of our study is the lower presence of smoking in subjects diagnosed at a younger age. In the study subjects, the frequency of never smokers under the age of 50 was close on 21%, whereas in those over the age of 50, the frequency was almost 10%. The prevalence of smoking falls even lower among subjects under the age of 35, with the frequency of never smokers being somewhat higher than 45% in this group. The results of Rich et al.,8 suggest something similar, on finding lower rates of tobacco-related tumors in the youngest group of subjects. This suggests that really young subjects with diagnosis of lung cancer may present with risk factors of the disease other than smoking, such as greater susceptibility or genetic predisposition.22 In addition to the differences present in genetic characteristics, there are other risk factors that should be borne in mind, such as occupational exposures, exposure to radon, environmental pollution, and socioeconomic level.22 Unfortunately, we have no data relating to other exposures of the subjects included, which might lead us to think of an alternative risk factor to tobacco use or genetic predisposition. It should be noted that previous studies have indeed associated exposure to radon with mutations in the EGFR gene and ALK translocation9 in a study conducted on never smokers, so that this hypothesis should not be ruled out.
In terms of genetic alterations, the results of this study agree with others carried out previously in other geographic contexts. A recent study conducted in the United Kingdom with 248 subjects under the age of 50 years found that the EGFR mutation and ALK translocation were present in 19% and 10% of the subjects, respectively.6 Furthermore, KRAS mutation was present in 12% of the subjects. Other studies conducted in Asia17,23 and America10 have reported similar results. That said, however, a study conducted in the Czech Republic found a higher proportion of mutation in EGFR in older subjects, whereas the contrary was observed for ALK translocations.4 Detection of the EGFR mutation appears to be associated with non-smokers, since different studies have found an inverse relationship between this mutation and smoking.24–26 This finding is also frequent in the Asian population, in which it is more usual to be a never smoker and there is a higher frequency of EGFR mutations.6 This agrees with what was observed in our study, in which most of the youngest subjects were never smokers and presented with this mutation. Furthermore, whereas the origin of certain mutations is linked to the carcinogens in tobacco (e.g., KRAS), the specific cause of EGFR mutations is not yet clear.27
Currently, a considerable number of genetic mutations have been identified and used to guide oncologic treatments.22 In recent decades, specific treatments targeted at EGFR, ALK and ROS1 alterations have been implemented, enhancing the survival of these patients. The scientific evidence suggests that, when the relevant genetic alterations are identified, the most effective treatment can be selected for each patient, thereby improving the clinical results. One study ascertained that, from the age of 50 years onwards, the incidence of these types of mutations decreases.10 Despite the fact that different studies have found worse survival in young subjects with lung cancer, a recent study has observed that survival of these subjects increased notably after receiving targeted treatment.6 The scientific evidence seems to support the need for personalized oncologic treatments in young subjects with diagnosis of lung cancer. Furthermore, identifying these mutations may be useful when it comes to developing new biomarkers that would allow for early detection of lung cancer in young subjects,28 not available in currently implemented screening programs.
This study has a series of advantages. One of them is the representativeness of all patients diagnosed with lung cancer in Spain, as previously reported.29 Secondly, the fact that there is universal health coverage in Spain is relevant, since it implies that the patients included, coming for the most part from public hospitals, properly represent the whole population. In addition to the abovementioned sample size. A further advantage lies in the fact that the cases included were all diagnosed from the end of 2016 onwards, which not only allows for good temporal comparability between these patients and those who are currently being treated elsewhere, but would also lead one to assume that there are no missing cases due to the absence of molecular analysis of the genes in question, something that does occur in the oldest cases series.
This study also has a series of limitations. Although the initial sample size is quite large (n=26,336), 4.5% were participants younger than 50 years and only 0.2% were younger than 35 years. It is important to note that most studies with a similar objective to ours also had a small sample size in patients younger than 50 years, as the incidence at these ages is very low. Apart from those already mentioned, i.e., not having data on participants’ occupations or exposure to radon, there are also no data on the possible role played by other human carcinogens, such as exposure to second-hand tobacco smoke or environmental pollution per se. Studying other risk factors for lung cancer would be interesting, especially because the prevalence of tobacco consumption is decreasing overall. Additionally, the cut-point of 50 years for differentiating young from ‘non-young’ subjects may seem arbitrary. It is true that there does not appear to be consensus when it comes to defining lung cancer in young subjects, given that some studies set the cut-point at 35 or 45 years. Even so, a recent study has identified 50 years as the age from which incidence of target genotypes decreases.10 A further limitation would be the potential selection or participation bias of the subjects in the TTR, however, in our opinion, it is unlikely as the participants were recruited by oncologists using consecutive sampling.
In conclusion, this study shows that patients diagnosed with lung cancer before the age of 50 years present with clinical and genetic characteristics different from those diagnosed at more advanced ages, including a greater predominance of women, never smokers, diagnosis at later stages, and a higher frequency of EGFR mutation and ALK translocation. These results highlight the importance of considering the individualized profile of each young patient with lung cancer, in order to offer personalized treatments. Furthermore, identification of genetic mutations associated with lung cancer in young subjects may improve the diagnosis of the disease in this age group. This may be important at health centers or in health systems where there may not be sufficient resources to make gene panels for all patients, suggesting that, in this context, their use in young patients should perhaps be prioritized, since it increases the likelihood of finding mutations that respond to specific treatments. Moreover, standardizing the performing of the genetic analysis across health centers is essential, given the existing heterogeneity among them.
Data AvailabilityThe data supporting the results of this work are available upon reasonable request (email: alberto.ruano@usc.es).
Ethics ApprovalThe Thoracic Tumors Registry was registered in ClinicalTrials.gov (NCT02942458), and the study protocol was approved by the institutional committee of the Puerta de Hierro University Teaching Hospital (Majadahonda, Madrid) (no. PI 148/15).
Authors’ ContributionsARR: conceptualization, methodology, supervision, writing-review and editing. MP: conceptualization, methodology, supervision, writing-review and editing. CCP: methodology, data curation, formal analysis, visualization, writing-original draft. The rest of the authors: investigation, resources, writing-review and editing.
FundingNone.
Conflicts of InterestsThe authors declare that there are no conflicts of interest with respect to the publication of this paper.
None.