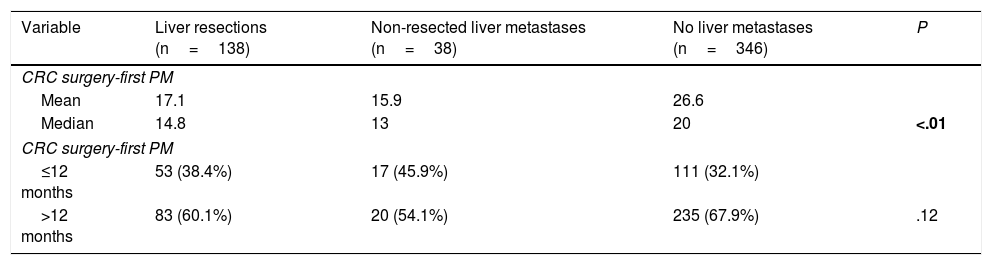Resection of both liver and lung metastases from colorectal carcinoma (CRC) is a standard of care in selected patients with oligometastatic disease. We present here the analysis of the subgroup of patients undergoing combined surgery from the Spanish Group of Surgery of Pulmonary Metastases (PM) from Colorectal Carcinoma (GECMP-CCR-SEPAR).
MethodsWe analyze characteristics, survival and prognostic factors of patients undergoing combined resection from March-2008 to February-2010 and followed-up during at least 3 years, from the prospective multicenter Spanish Registry.
ResultsA total of 138 patients from a whole series of 543 cases from 32 thoracic surgery units underwent both procedures. Seventy-seven (43.8%) resected liver metastases were synchronic with colorectal tumor. Median disease specific survival (DSS) from first pulmonary metastasectomy was 48.9 months, being three and 5-year DSS 65.1% and 41.7%, respectively. From CRC-surgery median DSS was 97.2 months, with 3 and 5-year DSS rates of 96.7% and 77%, respectively. Five-year DSS from pulmonary metastasectomy was 41.7% for patients with combined resection and 52.4% for those without hepatic involvement (P=.04). Differences disappeared when considering DSS from colorectal surgery. Carcinoembrionary antigen (CEA) before lung surgery over 10mg/dl and bilateral PM were independent prognostic factors for survival (hazard ratio 2.4 and 2.5, respectively).
ConclusionsPatients with resection of PM of CRC with history of resected hepatic metastases presented significantly lower disease specific survival rates than those undergoing pulmonary metastasectomy alone. CEA before lung surgery and bilateral PM associated worse prognosis.
La resección de metástasis hepáticas y pulmonares del carcinoma colorrectal (CCR) es un tratamiento estándar para determinados pacientes con enfermedad oligometastásica. Presentamos el análisis del subgrupo de pacientes sometidos a cirugía combinada del Grupo Español de Cirugía de Metástasis Pulmonares (MP) de Carcinoma Colorrectal (GECMP-CCR-SEPAR).
MétodosAnalizamos las características, la supervivencia y los factores pronósticos de los pacientes sometidos a resección combinada desde marzo de 2008 a febrero de 2010, con seguimiento durante al menos 3 años en el Registro Español, prospectivo y multicéntrico.
ResultadosSe sometieron a ambos procedimientos 138 pacientes de una serie completa de 543 casos, procedentes de 32 unidades de cirugía torácica. Setenta y siete (43,8%) de las metástasis hepáticas resecadas eran sincrónicas al tumor colorrectal. La mediana de la supervivencia específica para la enfermedad (SEE) desde la primera metastasectomía pulmonar fue de 48,9 meses; las SEE a 3 y 5 años fueron del 65,1 y 41,7%, respectivamente. La mediana de la SEE desde la cirugía del CCR fue de 97,2 meses, con tasas de la SEE a 3 y 5 años del 96,7 y 77%, respectivamente. Las SEE a 5 años de la metastasectomía pulmonar fueron del 41,7% para los pacientes con resección combinada y del 52,4% para aquellos sin afectación hepática (p=0,04). Las diferencias desaparecieron cuando se tenía en cuenta la SEE desde la cirugía colorrectal. Tener un nivel de antígeno carcinoembrionario (ACE) por encima de 10mg/dl antes de la cirugía pulmonar y la presencia bilateral de MP fueron factores pronósticos independientes para la supervivencia (odds ratio: 2,4 y 2,5, respectivamente).
ConclusionesLos pacientes con resección de MP de CCR con antecedentes de metástasis hepáticas resecadas presentaron tasas de SEE significativamente más bajas que aquellos sometidos a metastasectomía pulmonar sola. El ACE antes de la cirugía pulmonar y la presencia de MP bilaterales se asociaron a peor pronóstico.
Up to 70% of patients with colorectal carcinoma (CRC) present with metastases or will develop them throughout their disease,1 being liver and lung the main involved distant organs.2 Uncountable series of patients with resected pulmonary metastases (PM) of CRC report 5-year survival rates of around 50%.3 Classic criteria to consider pulmonary metastasectomy include feasibility of complete resection, ability of the patient to tolerate the procedure, control of primary tumor and absence of extra-thoracic disease (except resectable liver metastases). The identification of patients who really get benefit from surgery is not clear. Most of the published studies are single-center retrospective series with extended periods of collection, different criteria of inclusion and multimodal adjuvant treatment.4 Different prognostic factors have been advocated, but level of evidence in order to select patients to surgery is low.5 Some studies have shown patients with both liver and lung metastases during natural history of CRC disease who undergo R0 resection getting similar or slightly reduced overall survival compared to those with only pulmonary metastasectomy.6 However it has also been suggested that CRCs metastasizing liver and lung could present different oncologic profiles than those with isolated PM. Thus, it is worthwhile to analyze the behavior of cancer within this selected group of patients with combined metastases surgically treated instead of consider liver metastases as a simple one more factor within global series of pulmonary metastasectomy of CRC.7
The Spanish Society of Pneumology and Thoracic Surgery developed a cooperative group (GECMP-CCR-SEPAR) to show trends in surgery of PM of CRC in Spain. Several data from that investigation have already been published.8–13 We analyze here the cohort of patients undergoing surgical resection of PM of CRC with history of liver metastasectomy, focusing on characteristics of this subgroup, comparison to the whole series, survival and prognostic factors.
MethodsThe collaborative group developed by SEPAR included departments of thoracic surgery interested in participate in a national registry about surgery of metastases from colorectal carcinoma. Between members of the group, a task force established a scientific committee with the aim of deciding the aspects to be analyzed and the variables of the Registry. Then, Dynamic Solutions Company™ developed an online database. One member of every department was allowed to log in to prospectively include patients in the Registry. From the scientific committee, different groups were selected to analyze specific aspects from the Registry. Management of data and Statistical analysis were all performed by Dynamic Solutions™ based on the requests of the different groups. Participation of the highest number of centers in order to reach the goal number of patients with a period of inclusion as short as possible was a main goal. Thirty two thoracic surgery departments actively included patients in the Registry. Approval of the Research and Ethics Committees of the centers was collected. Patients underwent first pulmonary metastasectomy with radical intent from March-2008 to February-2010. The Registry included different sections: general demographic variables, data of primary tumor, first and successive episodes of pulmonary metastasectomy, locorregional or extrathoracic disease, and follow-up. Criteria for deciding the use of chemotherapy and the moment of indication of resection were not homogeneous. Data about regiments of chemotherapy used were not collected. Data regarding associated liver metastases could be registered at three points within the Registry: present at the time of the primary resection (primary tumor section), emerging after primary resection without lung disease (extrathoracic disease section) or synchronic to PM (in his corresponding pulmonary metastasectomy episode section). Metastases were considered synchronic when time between diagnoses of both lesions was less than three months.
Differences in characteristics between the subgroup of patients undergoing combined resections and the rest of series were analyzed with Fisher's exact test for non continuous variables and Kruskal–Wallis test for continuous variables, such as number of PM resected and disease free interval. In-pairs comparisons of three cohorts in cases of continuous variables were performed with Mann–Whitney statistical test. We assessed differences regarding pattern of appearance of liver metastases (synchronous/metachronous) using Fisher's exact test. Non-surgical radical treatments of liver metastases were also analyzed, being considered non resected patients. All the patients were followed for at least three years from first pulmonary metastasectomy. At least a thoracoabdominal computed tomography (CT) every six months was performed. For survival analysis (Kaplan–Meier), disease-related deaths and recurrences were considered events, and patients with either non disease-related deaths or alive without recurrence were censored at the last follow-up. Losses were recorded as censored cases. For survival assessment three cohorts of patients were mutually compared: (a) patients undergoing combined liver and lung metastasectomy, (b) patients with liver metastases not amenable to be resected, and (c) patients without hepatic metastases within the period of analysis. We analyzed disease specific survival (DSS) from colorectal surgery and from first pulmonary metastasectomy. Also recurrence free survival (RFS) after lung surgery was assessed. Relapse of the disease was defined as the presence of a suggestive image. Histological confirmation was not mandatory.
A prognostic model of the whole series was developed as the first endpoint of the Registry.10 We tried to define independent prognostic factors in the cohort of patients with combined liver and lung resections. This study is reported according to STROBE statement.
A sample size of 512 patients was established. It was calculated in order to detect risk factors with a hazard ratio ≥1.8, with α error of 5% and a power of 80%. A total of 15% of losses were assumed. Five-year overall survival (OS) fewer than 50% was predicted. Statistical significance was assessed by two-tailed test. For the bivariant analysis, age was dichotomized (cutoff 65-years), and value of serum levels of carcinoembrionary antigen (CEA) was classified into three groups (cutoffs: 5 and 10mg/dl). Cutoffs of 12 and 24 months were established for RFS. Survival analysis was performed using Kaplan–Meier method, showing mean, median and 95% confidence intervals (CI). Comparison of survival curves was performed with Log-Rank test. Variables found to be significant on univariate analysis (P<.2) were included in the multivariate analysis using the Cox proportional hazards model, showing hazard ratios (HR) and 95% confidence intervals. SPSS 22.0® (IBM Inc. Chicago, IL) was used.
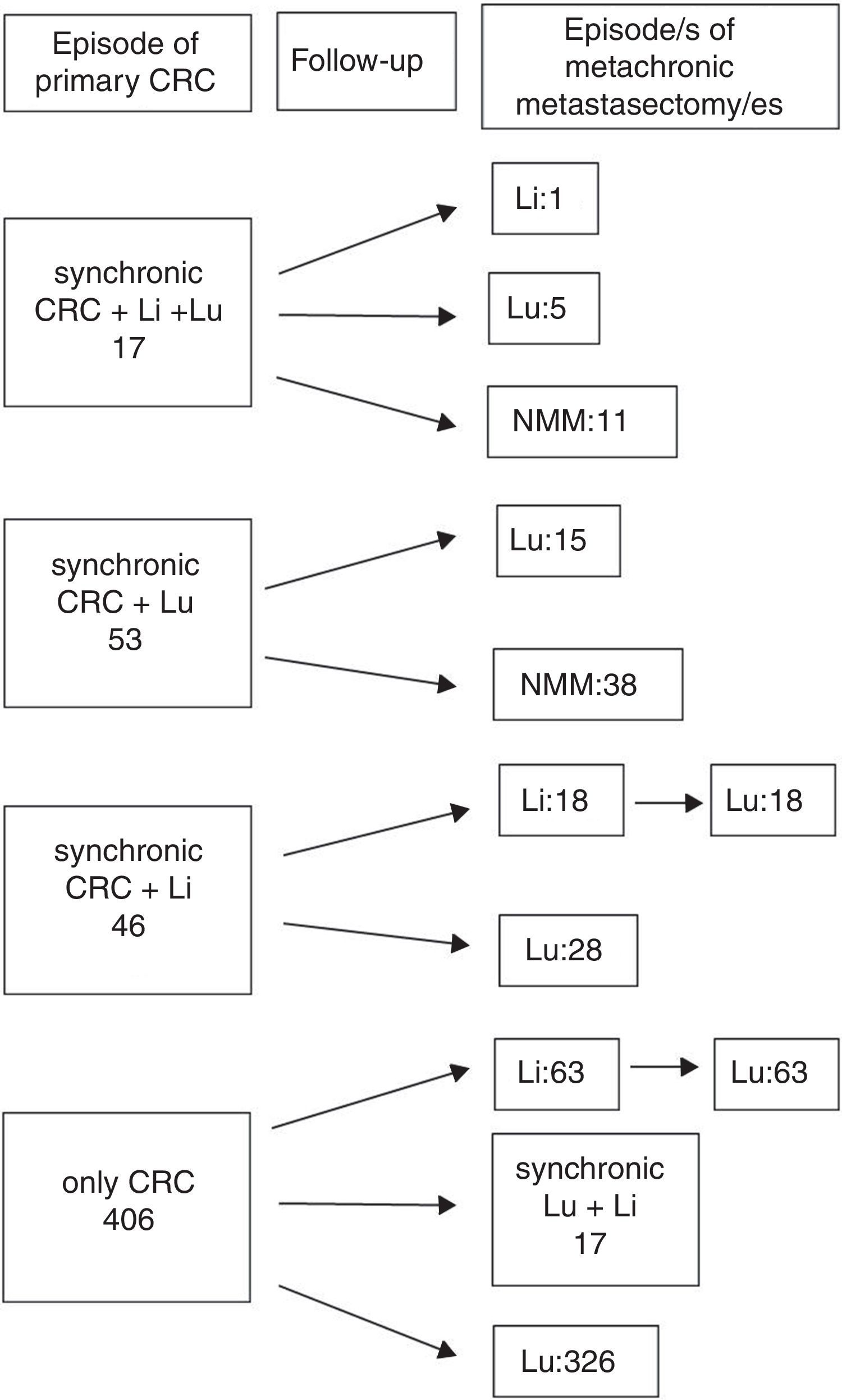
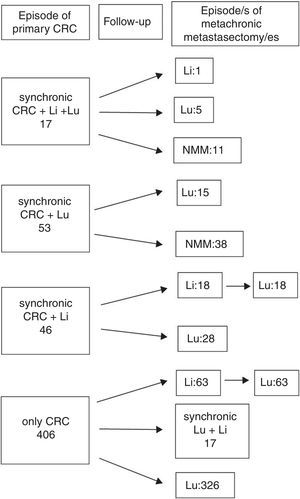
ResultsA total 543 patients were included. Twenty-one cases (4%) did not meet all the inclusion criteria, being 522 patients eventually analyzed, 176 (33.7%) presenting with one or more liver metastases at any time. Hepatic resections were performed in 138 (78.4%), and 38 patients presented with liver metastases after pulmonary metastasectomy and were not radically treated. A flowchart summarizing surgical history of colorectal metastatic disease is shown in Fig. 1: In 17 patients, colorectal, hepatic and pulmonary involvement was synchronic. Among them, one patient underwent a new liver resection, five patients did lung resection and 11 patients did not undergo new metastasectomies during the follow-up. Fifty three patients presented with synchronic CRC and PM (15 of them with a new episode of resected metachronic PM), and 46 patients presented with synchronic CRC and hepatic involvement. Among the latter, 18 patients underwent liver and lung metastasectomies, and 28 only pulmonary resections. The remaining 406 patients presented with metachronic disease during the follow-up after CRC surgery: 80 with combined resection and 326 with pulmonary resection alone. In six cases pulmonary metastasectomy was previous to hepatic resection. Median interval between hepatic and pulmonary resection was 19.5 months. One patient underwent two episodes of liver resection.
Therapeutical evolution of colorrectal metastatic disease for the global series. Values refers to metastasis treated with surgery. Sequential abdominal/thoracic surgical procedures of synchronic liver and lung metastases are considered coincidental. n=522 patients. CRC: colorectal, Li: liver, Lu: lung, NMM: no more episodes of metastasectomy.
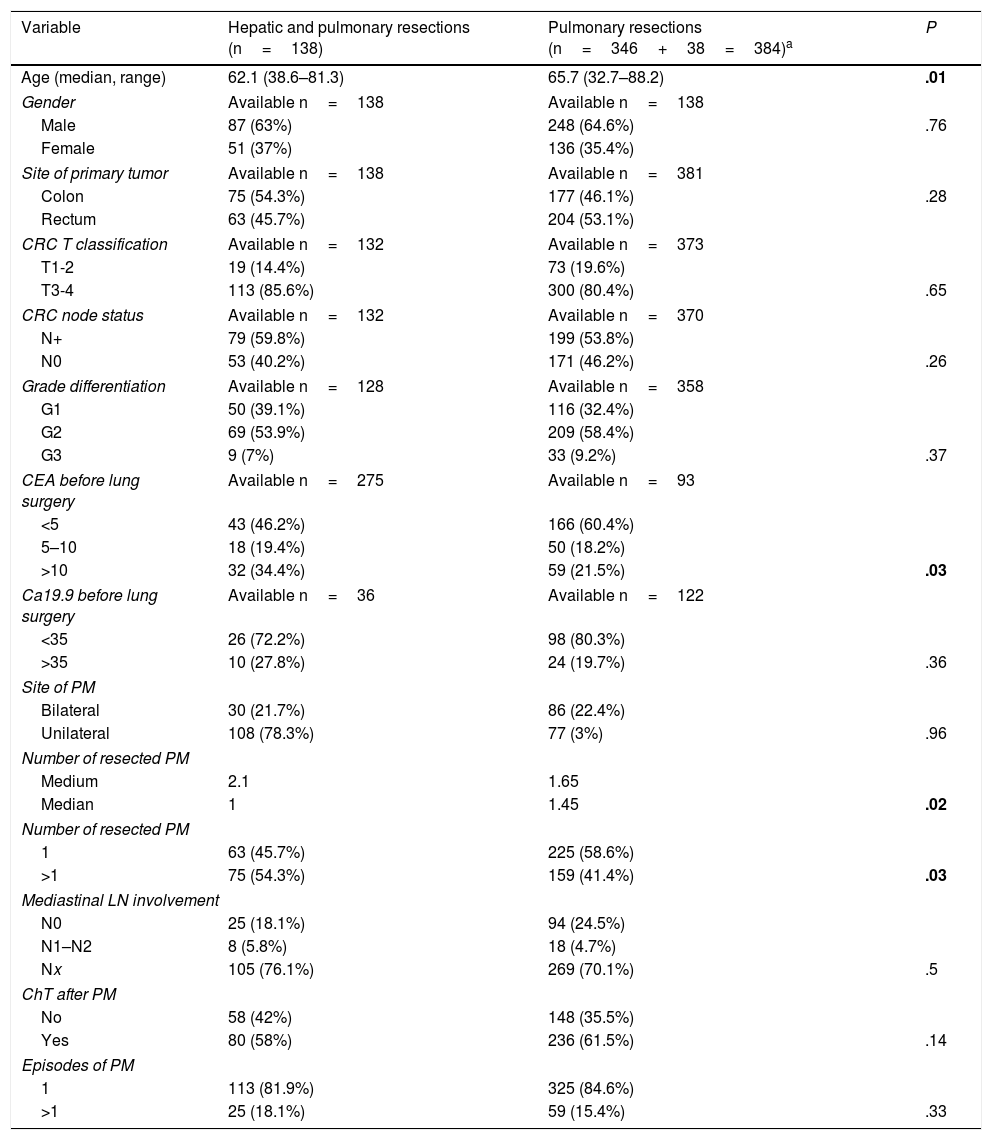
Clinical and surgical characteristics of the whole series have been previously published.9,11,13 Characteristics of the patients with combined lung and liver resections and comparison with those without liver involvement are summarized in Table 1. Patients undergoing only pulmonary resection were older than those with combined surgery. There were no differences related to site of primary tumor, T stage, N involvement or grade of differentiation. Levels of CEA determined within 30 days before pulmonary metastasectomy were higher among patients with combined resections. Lung metastases were multiple in more cases in the combined surgery group. No differences were found regarding mediastinal lymph node (LN) involvement, adjuvant chemotherapy, or number of episodes of pulmonary resection.
Characteristics of Patients With Combined Resections. Comparison to the Remaining Patients of the Registry.
| Variable | Hepatic and pulmonary resections (n=138) | Pulmonary resections (n=346+38=384)a | P |
|---|---|---|---|
| Age (median, range) | 62.1 (38.6–81.3) | 65.7 (32.7–88.2) | .01 |
| Gender | Available n=138 | Available n=138 | |
| Male | 87 (63%) | 248 (64.6%) | .76 |
| Female | 51 (37%) | 136 (35.4%) | |
| Site of primary tumor | Available n=138 | Available n=381 | |
| Colon | 75 (54.3%) | 177 (46.1%) | .28 |
| Rectum | 63 (45.7%) | 204 (53.1%) | |
| CRC T classification | Available n=132 | Available n=373 | |
| T1-2 | 19 (14.4%) | 73 (19.6%) | |
| T3-4 | 113 (85.6%) | 300 (80.4%) | .65 |
| CRC node status | Available n=132 | Available n=370 | |
| N+ | 79 (59.8%) | 199 (53.8%) | |
| N0 | 53 (40.2%) | 171 (46.2%) | .26 |
| Grade differentiation | Available n=128 | Available n=358 | |
| G1 | 50 (39.1%) | 116 (32.4%) | |
| G2 | 69 (53.9%) | 209 (58.4%) | |
| G3 | 9 (7%) | 33 (9.2%) | .37 |
| CEA before lung surgery | Available n=275 | Available n=93 | |
| <5 | 43 (46.2%) | 166 (60.4%) | |
| 5–10 | 18 (19.4%) | 50 (18.2%) | |
| >10 | 32 (34.4%) | 59 (21.5%) | .03 |
| Ca19.9 before lung surgery | Available n=36 | Available n=122 | |
| <35 | 26 (72.2%) | 98 (80.3%) | |
| >35 | 10 (27.8%) | 24 (19.7%) | .36 |
| Site of PM | |||
| Bilateral | 30 (21.7%) | 86 (22.4%) | |
| Unilateral | 108 (78.3%) | 77 (3%) | .96 |
| Number of resected PM | |||
| Medium | 2.1 | 1.65 | |
| Median | 1 | 1.45 | .02 |
| Number of resected PM | |||
| 1 | 63 (45.7%) | 225 (58.6%) | |
| >1 | 75 (54.3%) | 159 (41.4%) | .03 |
| Mediastinal LN involvement | |||
| N0 | 25 (18.1%) | 94 (24.5%) | |
| N1–N2 | 8 (5.8%) | 18 (4.7%) | |
| Nx | 105 (76.1%) | 269 (70.1%) | .5 |
| ChT after PM | |||
| No | 58 (42%) | 148 (35.5%) | |
| Yes | 80 (58%) | 236 (61.5%) | .14 |
| Episodes of PM | |||
| 1 | 113 (81.9%) | 325 (84.6%) | |
| >1 | 25 (18.1%) | 59 (15.4%) | .33 |
CRC: colorectal; PM: pulmonary metastases; LN: lymph node; ChT: chemotherapy.
Bold P values refers to significance.
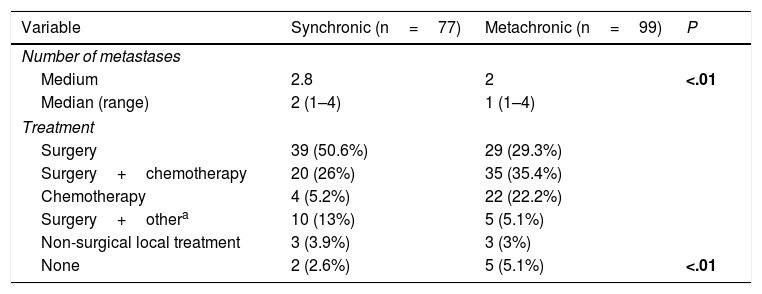
A total of 77 liver metastases (43.8%) were synchronic with CRC. Number of hepatic metastases was higher in cases of synchronic disease (P<.01). Eight patients with synchronic liver metastases were managed with non-surgical local therapeutic tools (Table 2).
Number of Liver Metastases and Treatment Regarding Pattern of Appearance.
| Variable | Synchronic (n=77) | Metachronic (n=99) | P |
|---|---|---|---|
| Number of metastases | |||
| Medium | 2.8 | 2 | <.01 |
| Median (range) | 2 (1–4) | 1 (1–4) | |
| Treatment | |||
| Surgery | 39 (50.6%) | 29 (29.3%) | |
| Surgery+chemotherapy | 20 (26%) | 35 (35.4%) | |
| Chemotherapy | 4 (5.2%) | 22 (22.2%) | |
| Surgery+othera | 10 (13%) | 5 (5.1%) | |
| Non-surgical local treatment | 3 (3.9%) | 3 (3%) | |
| None | 2 (2.6%) | 5 (5.1%) | <.01 |
Bold P values refer to significance.
A total of 119 patients (86.2%) received adjuvant chemotherapy after resection of CRC. One patient did not receive adjuvance and data were not available for the remaining 18 patients. Nineteen patients (13.8%) received adjuvant radiotherapy.
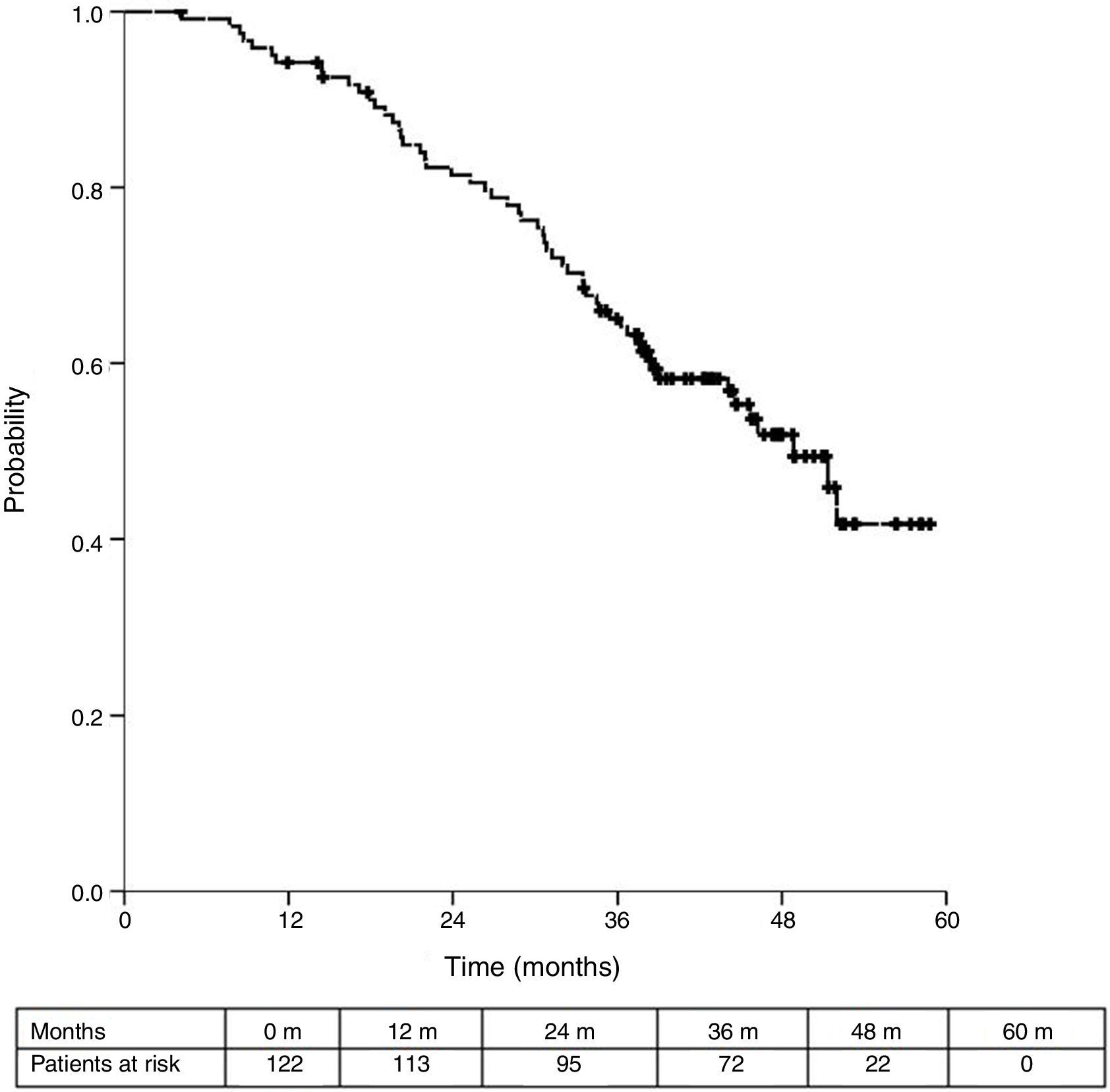
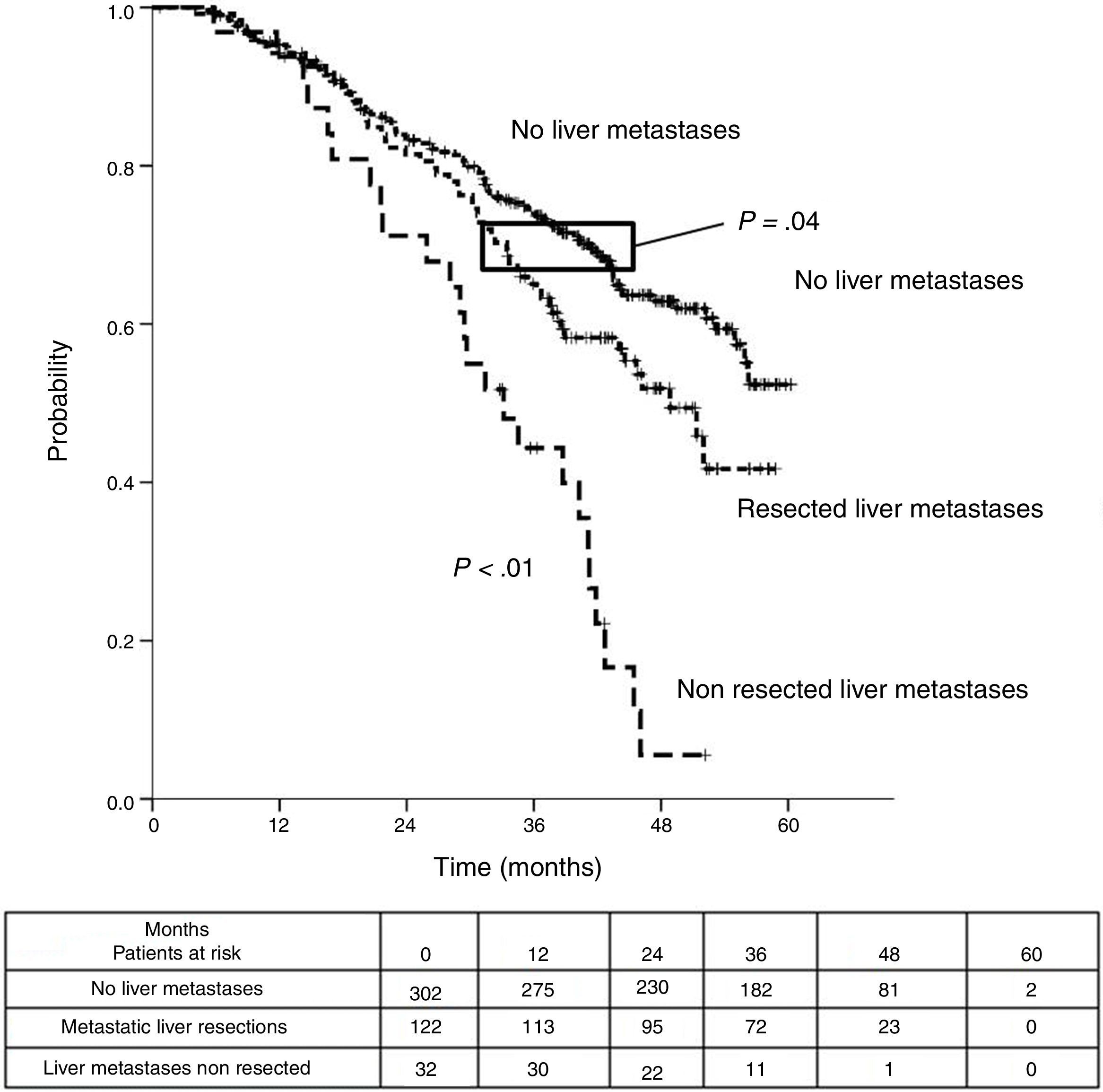
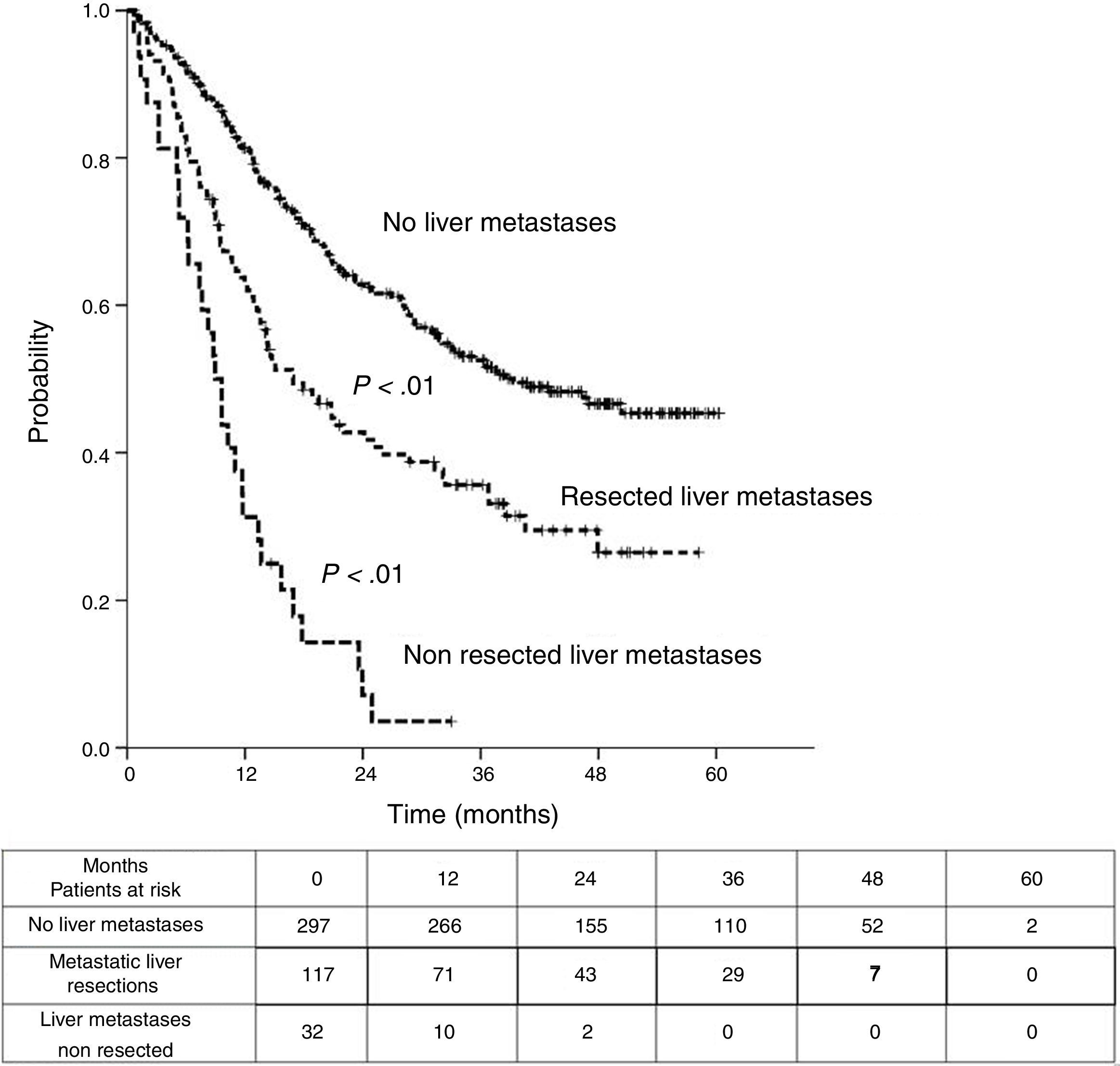
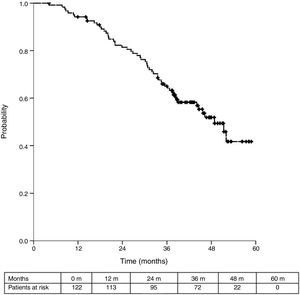
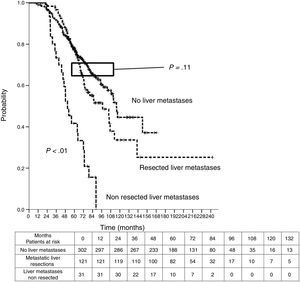
Sixteen patients (11.6%) were excluded from the survival analysis due to lack of follow-up data. Among 122 valid cases (whole series), a total of 55 events were recorded after a mean follow-up of 55.8 months after liver metastasectomy and 36 months after first pulmonary metastasectomy. Within 32 patients with non-resected hepatic involvement, 25(78.1%) events had happened at the end of follow-up. Median DSS from first pulmonary metastasectomy (mean follow-up of 36 months) was 48.9 months (95%CI 43–54.7). Three and 5-year DSS were 65.1% (95%CI 56.4–73.7) and 41.7% (95%CI 28.3–55.1) respectively (Fig. 2).
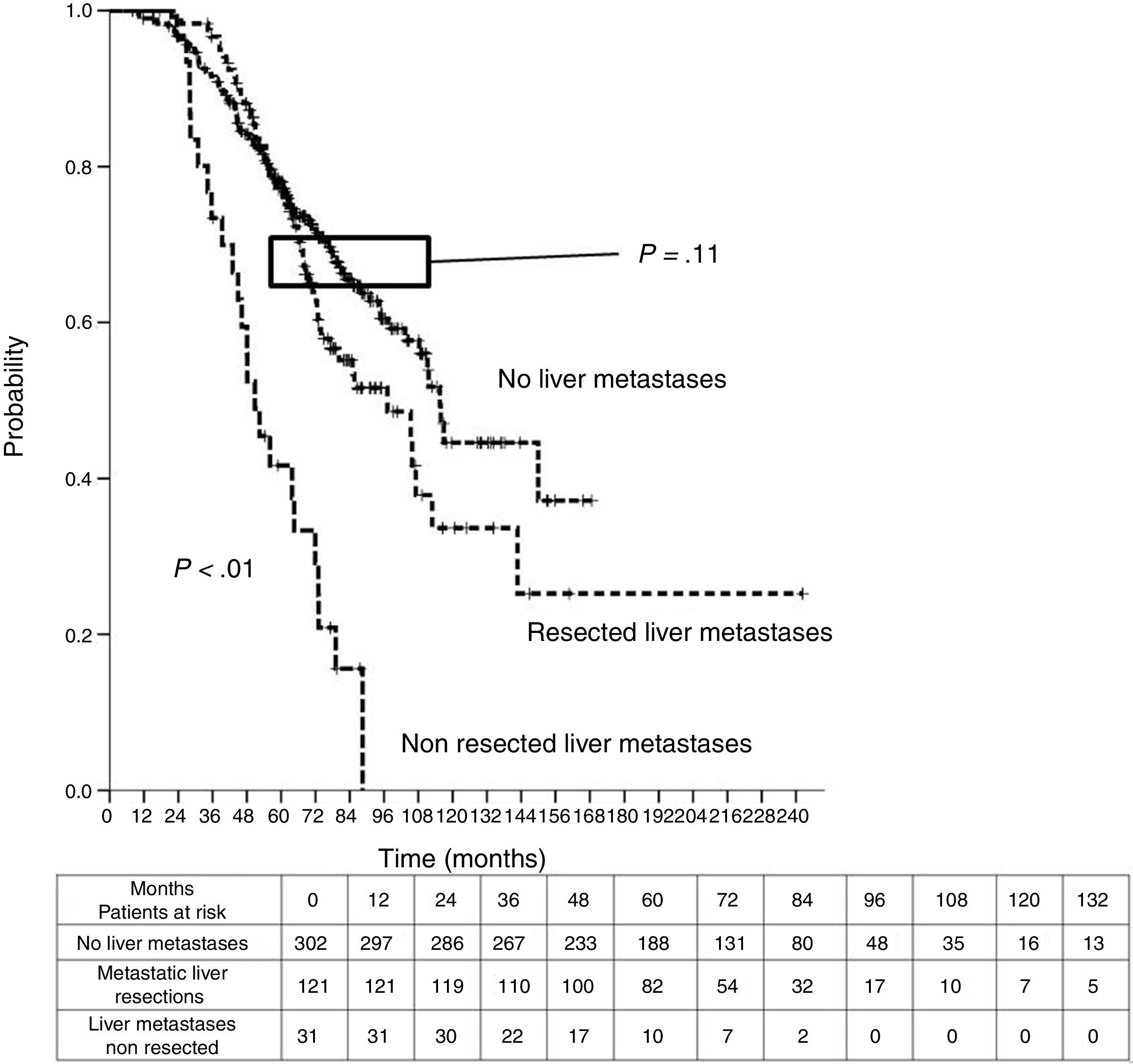
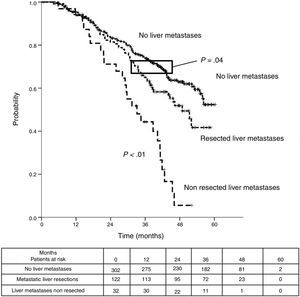
Median DSS from liver metastasectomy was 74 months (95%CI 60.2–87.8). Three and 5-year DSS were 83.6% (95% CI 76.9%–90.4%) and 63% (95%CI 53.9%–72%) respectively. Finally, from CRC, after a mean follow-up of 72.9 months, median DSS was 97.2 months (95%CI 75.6–118.8). Three and 5-year DSS were 96.7% (95%CI 93.4%–100%) and 77% (95%CI 69.3%–84.8%) respectively.
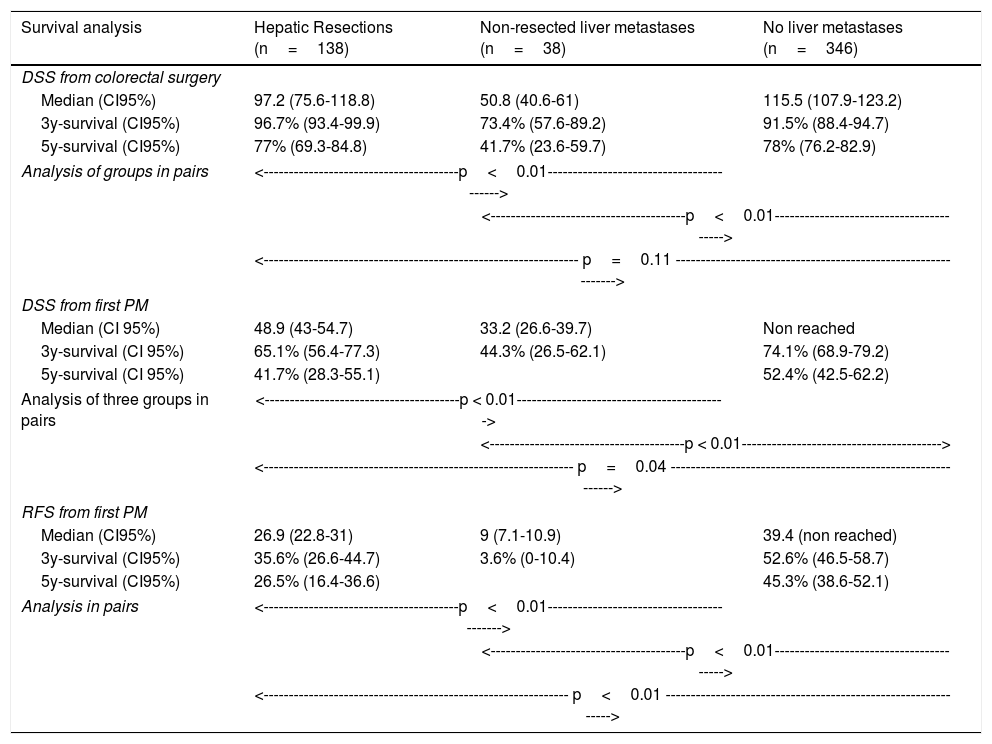
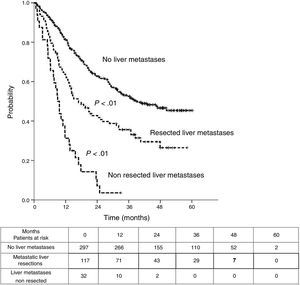
Table 3 shows comparison of survival among the three cohorts analyzed. DSS after colorectal surgery (Fig. 3) and after first pulmonary metastasectomy (Fig. 4) were evaluated. Cohorts were compared in pairs (log-rank). Setting the starting-point at the moment of first pulmonary metastasectomy, 5-year DSS from pulmonary resection was lower among patients undergoing combined metastasectomy (41.7%) than those without hepatic involvement (52.4%) (P=.04). However differences disappeared when considering DSS from colorectal surgery, with 5-year survival of 77% and 78% respectively. RFS after first pulmonary resection was also significantly lower in patients undergoing liver resection than among those with lung surgery only (Fig. 5). Finally, poor prognosis was proven for non-resected patients with hepatic metastases, with 3-year survival after first thoracic surgery of 44.3% (95% CI 26.5–62.1).
Survival analysis of three cohorts: no liver involvement, resected liver metastases and non-resected liver metastases. In pairs comparison between groups using log-rank test.
| Survival analysis | Hepatic Resections (n=138) | Non-resected liver metastases (n=38) | No liver metastases (n=346) |
|---|---|---|---|
| DSS from colorectal surgery | |||
| Median (CI95%) | 97.2 (75.6-118.8) | 50.8 (40.6-61) | 115.5 (107.9-123.2) |
| 3y-survival (CI95%) | 96.7% (93.4-99.9) | 73.4% (57.6-89.2) | 91.5% (88.4-94.7) |
| 5y-survival (CI95%) | 77% (69.3-84.8) | 41.7% (23.6-59.7) | 78% (76.2-82.9) |
| Analysis of groups in pairs | <---------------------------------------p<0.01-----------------------------------------> | ||
| <---------------------------------------p<0.01----------------------------------------> | |||
| <--------------------------------------------------------------- p=0.11 --------------------------------------------------------------> | |||
| DSS from first PM | |||
| Median (CI 95%) | 48.9 (43-54.7) | 33.2 (26.6-39.7) | Non reached |
| 3y-survival (CI 95%) | 65.1% (56.4-77.3) | 44.3% (26.5-62.1) | 74.1% (68.9-79.2) |
| 5y-survival (CI 95%) | 41.7% (28.3-55.1) | 52.4% (42.5-62.2) | |
| Analysis of three groups in pairs | <---------------------------------------p < 0.01------------------------------------------> | ||
| <---------------------------------------p < 0.01----------------------------------------> | |||
| <-------------------------------------------------------------- p=0.04 --------------------------------------------------------------> | |||
| RFS from first PM | |||
| Median (CI95%) | 26.9 (22.8-31) | 9 (7.1-10.9) | 39.4 (non reached) |
| 3y-survival (CI95%) | 35.6% (26.6-44.7) | 3.6% (0-10.4) | 52.6% (46.5-58.7) |
| 5y-survival (CI95%) | 26.5% (16.4-36.6) | 45.3% (38.6-52.1) | |
| Analysis in pairs | <---------------------------------------p<0.01------------------------------------------> | ||
| <---------------------------------------p<0.01----------------------------------------> | |||
| <------------------------------------------------------------- p<0.01 --------------------------------------------------------------> | |||
DSS: disease specific survival, RFS: recurrence free survival, PM: pulmonary metastasectomy, CI: confidence interval
Interval between colorectal surgery and first pulmonary metastasectomy was significantly lower in patients with history of liver metastasectomy (14.8 versus 20 months) (Table 4).
Intervals Between CRC Surgery and First PM.
| Variable | Liver resections (n=138) | Non-resected liver metastases (n=38) | No liver metastases (n=346) | P |
|---|---|---|---|---|
| CRC surgery-first PM | ||||
| Mean | 17.1 | 15.9 | 26.6 | |
| Median | 14.8 | 13 | 20 | <.01 |
| CRC surgery-first PM | ||||
| ≤12 months | 53 (38.4%) | 17 (45.9%) | 111 (32.1%) | |
| >12 months | 83 (60.1%) | 20 (54.1%) | 235 (67.9%) | .12 |
CRC: colorectal, PM: pulmonary metastasectomy.
Bold P value refers to significance.
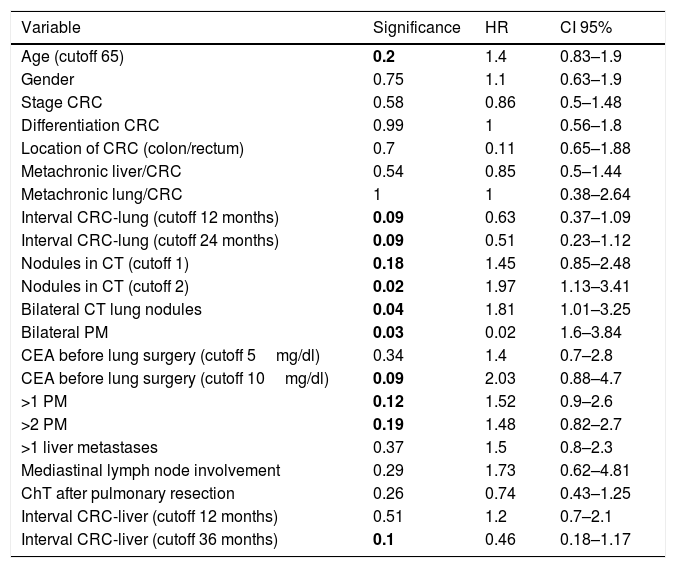
Univariate analysis is shown in Table 5. Variables with P value≤.2 were selected for the multivariate analysis (Cox model). Two variables were finally proven as independent prognostic factors: CEA before lung surgery over 10mg/dl: HR 2.38 (CI 95%1–5.7), and bilateral PM: HR 2.5 (CI 95%1.1–5.8), with P values of .05 and .03, respectively (Table 6).
Univariant Analysis of Potential Prognostic Factors.
| Variable | Significance | HR | CI 95% |
|---|---|---|---|
| Age (cutoff 65) | 0.2 | 1.4 | 0.83–1.9 |
| Gender | 0.75 | 1.1 | 0.63–1.9 |
| Stage CRC | 0.58 | 0.86 | 0.5–1.48 |
| Differentiation CRC | 0.99 | 1 | 0.56–1.8 |
| Location of CRC (colon/rectum) | 0.7 | 0.11 | 0.65–1.88 |
| Metachronic liver/CRC | 0.54 | 0.85 | 0.5–1.44 |
| Metachronic lung/CRC | 1 | 1 | 0.38–2.64 |
| Interval CRC-lung (cutoff 12 months) | 0.09 | 0.63 | 0.37–1.09 |
| Interval CRC-lung (cutoff 24 months) | 0.09 | 0.51 | 0.23–1.12 |
| Nodules in CT (cutoff 1) | 0.18 | 1.45 | 0.85–2.48 |
| Nodules in CT (cutoff 2) | 0.02 | 1.97 | 1.13–3.41 |
| Bilateral CT lung nodules | 0.04 | 1.81 | 1.01–3.25 |
| Bilateral PM | 0.03 | 0.02 | 1.6–3.84 |
| CEA before lung surgery (cutoff 5mg/dl) | 0.34 | 1.4 | 0.7–2.8 |
| CEA before lung surgery (cutoff 10mg/dl) | 0.09 | 2.03 | 0.88–4.7 |
| >1 PM | 0.12 | 1.52 | 0.9–2.6 |
| >2 PM | 0.19 | 1.48 | 0.82–2.7 |
| >1 liver metastases | 0.37 | 1.5 | 0.8–2.3 |
| Mediastinal lymph node involvement | 0.29 | 1.73 | 0.62–4.81 |
| ChT after pulmonary resection | 0.26 | 0.74 | 0.43–1.25 |
| Interval CRC-liver (cutoff 12 months) | 0.51 | 1.2 | 0.7–2.1 |
| Interval CRC-liver (cutoff 36 months) | 0.1 | 0.46 | 0.18–1.17 |
HR: hazard ratio, CRC: colorectal carcinoma, CRC: colorectal, CT: computed tomography, CEA: carcinoembrionary antigen, PM: pulmonary metastases, ChT: chemotherapy.
Bold P values refer to significance.
Combined surgical resection of liver and lung metastases of CRC in patients has become common practice as therapeutic strategy in the so called oligometastatic patient.14 Many surgical retrospective series have shown 5-year OS rates for patients with resected lung and liver metastases from CRC of 30%–60% after pulmonary metastasectomy.15 These series usually suffer limitations such as highly selected patients, small size of the data set and long inclusion periods. Recently Salah et al., have published a pooled analysis after searching the PubMed database, including 146 patients from five studies.16 Our cohort from the prospective Spanish Registry includes 138 patients undergoing both liver and lung resections from CRC from a whole series of 543 patients with resected lung metastases over a two years period.9 As far as we know it is the greatest surgical series of patients with liver and lung metastasectomy ever published. Some results referring different topics from the whole series area available.9–13
From our whole series, 24.4% of patients underwent liver metastasectomy (95.7% before lung surgery). Comparing to those undergoing only lung surgery, patients were younger and both CEA before lung surgery and number of resected PM were higher. No differences were found regarding CRC site, stage or differentiation. Mediastinal LN involvement, episodes of pulmonary metastasectomy and type of surgical resection were also similar. Hattori et al. do not find differences between patients undergoing lung versus combined resection regarding age, gender, site of primary tumor, CEA or number of metastases.17 Brouquet or Marin select their cohorts of combined surgery from a global series of liver metastasectomy.18,19 In our study, the whole series of patients undergoing resection of PM included 38 patients with non-resected liver metastases. From them, eight were synchronous, being treated with radical purpose, and 30 patients presented metachronous non-resected liver metastases, all of them after pulmonary surgery. The role of non-surgical radical treatments was out of the aim of this study. We decided to include those patients into the subgroup of non-resected liver metastases, in order to avoid biases in the main cohort of our study, given the great heterogeneity among different series dealing surgery of metastases.1 Terms synchronic and metachronic are not well defined.20–22 Interoperative interval or a synchronic diagnosis of M1 disease use not to be determinant when making a decision to indicate metastasectomies.23 We considered liver and lung metastases as synchronic when time between diagnosis of both lesions was under three months as other authors have published.6,24 Interval between colorectal surgery and first lung metastasectomy was significantly longer among patients without liver involvement.25 This fact may support a higher activity of the disease among patients with more than one distant organ involved.
As previously published, postoperative mortality after pulmonary resection was low.13 Only deaths due to oncological reasons were accounted as events. A precise cause of death may be difficult to establish and no fixed rules were developed in order to standardize this variable.
The moment from which OS is analyzed varies depending on the nature of the cohort evaluated. Most papers usually establish the time of thoracic surgery as the starting point. However they also use to include survival from colorectal surgery. Three and 5-year DSS from the whole series of the Registry were 69.4% and 46.1% respectively as it has been published.6 Patients with non-resected liver metastases showed a significantly poorer prognosis, independently of the starting point, as Limmer et al. find in their series.26 In our study DSS from colorectal surgery among patients with combined resections was similar to that of patients with only thoracic surgery. From first pulmonary metastasectomy, both DSS and RFS were significantly higher among patients with only lung metastases than those undergoing combined surgery. This find would somehow reflect that the presence of both liver and pulmonary disease throughout the follow-up of CRC does not influence final prognosis if complete resection can be achieved. However, if we sight survival curves after colorectal surgery (Fig. 3) we could guess that differences in survival would probably become apparent from five years onwards.
Whether good prognosis of these patients is a result of surgical procedures or a selection of patients with favorable disease itself (oligometastatic condition) remains unsettle.14 When a decision about the indication of resection of PM must be made, history of liver metastases should be taken into account as a prognostic factor. However, it should not contraindicate lung resections itself, because in default of a prospective randomized trial that lighten the role of surgery in this setting, the fact is that prognosis of patients with non-resected liver involvement is fateful. Five-year-DSS after lung metastasectomy in patients with resected liver metastases was 41.7%, similarly to other studies published during last two decades.17,27,28 Aforementioned metaanalysis of Salah shows an encouraging 5-year-OS of 54.4%.16 Other reports show similar 5-year-OS after pulmonary metastasectomy for patients with combined surgery. Andres et al. report 5-year-DSS for patients undergoing resection of synchronic liver and lung metastases of 40.7%.6
We found 5-year-RFS of 45.3% and 26.5% in patients with only lung metastases and combined surgery, respectively. RFS may be a biased parameter, because it depends on the intensity of surveillance during the follow-up.
CEA before lung surgery over 10mg/dl and bilateral PM were bad prognostic factors in the multivariate analysis. CEA was proven as prognostic factor in a few similar series,25,29 although Nojiri et al., or the metaanalysis of Salah could not confirm that relation.16,28 In our cohort synchronic disease and number of pulmonary nodules were not associated to worse prognosis, these findings being consistent with Salah, Zabaleta or Sha.16,22,30 Mediastinal LN involvement was not a prognostic factor in our series either. However, LN involvement was the only parameter that correlated with decreased survival for Salah et al., who advocates preoperative mediastinal staging to avoid futile surgical approaches.16 Administration of chemotherapy around liver resection did not carry differences in prognosis. However patients with neoadjuvant chemotherapy and those with postoperative chemotherapy were not analyzed separately. In the whole series, neoadjuvant chemotherapy before lung resection associated with worse prognosis, whilst adjuvance did not.10
Our Registry has important limitations. First, only patients undergoing surgical resection of PM are included. A surgical series defines a highly selected cohort of patients. Without prospective trials comparing those patients with similar cases not undergoing surgery, we cannot establish evidence-based recommendations.14,31 Moreover, pattern of recurrences after resection of lung metastases was not analyzed. Second, we only analyzed adjuvant chemotherapy after PM. Other studies assess the role of chemotherapy at different times throughout disease. Heterogeneity of regimens makes it impossible to draw conclusions about the effect of chemotherapy on survival. Third, anatomical location of primary colorectal tumor would have been more precise analyzing separately tumors of distal rectum. Finally, neither indication for metastasectomy nor the decision of using other treatments obeyed tight criteria.
ConclusionsPatients with PM of CRC with history of resected liver metastases presented significantly lower DSS rates from lung metastasectomy, but no from surgery of primary cancer, when comparing with those with only pulmonary metastasectomy. Among patients undergoing combined resection, CEA before lung surgery over 10mg/dl and bilateral PM were the only independent prognostic factors. Prospective well-designed studies are necessary. Until then, classical criteria for surgery of patients with metastatic CRC remain in force.
Conflict of InterestThe authors declare no conflict of interest.
The Members of the Spanish Group of Lung Metastases of ColoRectal Cancer (GECMP-CCR) of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR): Coordinators: Juan J. Rivas, Laureano Molins. Secretary: Raul Embun. Local heads: Francisco Rivas-Doyague, Raul Embun, Jorge Hernandez-Ferrández, Félix Heras, Javier de la Cruz, Matilde Rubio, Esther Fernández, Miguel Carbajo, Rafael Peñalver, José R. Jarabo, Diego González-Rivas, Sergio Bolufer, Carlos Pages, Sergi Call, David Smith, Richard Wins, Antonio Arnau, Andrés Arroyo, Carmen Marrón, Akiko Tamura, Montse Blanco, Beatriz de Olaiz, Gemma Munoz, José M. García Prim, Carlos Rombolá, Santiago García-Barajas, Alberto Rodríguez-Fuster, Jorge L. Freixinet, Javier Ruiz-Zafra, Guillermo Carriquiry, Moisés Rosenberg, Emilio Canalís.