Patients admitted to the intensive care unit (ICU) are at high risk of developing peripheral and respiratory muscle compromise.1–3 ICU-acquired limb muscle weakness (ICUAW) and ventilator-induced diaphragm dysfunction (VIDD) are the hallmarks of myopathy affecting critically ill patients. The presence of either ICUAW or VIDD is associated with poorer outcomes, including prolonged mechanical ventilation, ICU stay and mortality.1,2,4–8 Muscle wasting plays a central role in the development of skeletal muscle weakness. Limb and respiratory muscle atrophy occurs rapidly in critically ill patients.9,10 Few studies have analyzed the development of ICUAW and VIDD in the same subjects, and reports describing the evolutionary pattern of muscle wasting affecting both peripheral and respiratory muscles in critically ill patients are scarce.2,11–15 Dres et al. demonstrated that limb muscle and severe diaphragm weakness have a cumulative negative impact on patients’ outcome.12 Whether peripheral muscle and diaphragm atrophy also have a cumulative impact is unknown.
The aim of this pilot study was to describe the evolutionary pattern of limb muscle and diaphragm thickness in critically ill patients requiring mechanical ventilation, and to determine whether the combination of limb and diaphragmatic atrophy has a cumulative impact on patients’ outcome.
Thirty-two adult patients admitted to ICU and requiring invasive mechanical ventilation were included. Diaphragm, mid-upper arm, mid-forearm and mid-thigh muscle thickness were measured by ultrasound on admission and repeated on days three and seven (Supplementary Fig. 1). Diaphragmatic atrophy was defined as a decrease in diaphragm thickness ≥5% from baseline. Limb muscle atrophy was considered when any of the peripheral muscles had a decrease in thickness ≥5% from baseline. Subjects associating diaphragmatic and limb muscle atrophy were considered to present combined muscle atrophy.
Categorical variables were reported as absolute numbers (percentage) and compared using Chi-square test or Fisher exact test. Continuous variables were expressed as mean±standard deviation if normally distributed, or median (25th, 75th percentile) if not. Student t test was used to compare initial muscle thickness between groups. Mann-Whitney U test was performed to compare muscle thickness change from baseline between atrophy and no-atrophy groups at each time point. The Spearman correlation was used to analyze bivariate correlations. A P value<0.05 was considered statistically significant. A more detailed description of the methods is provided in the Supplementary material.
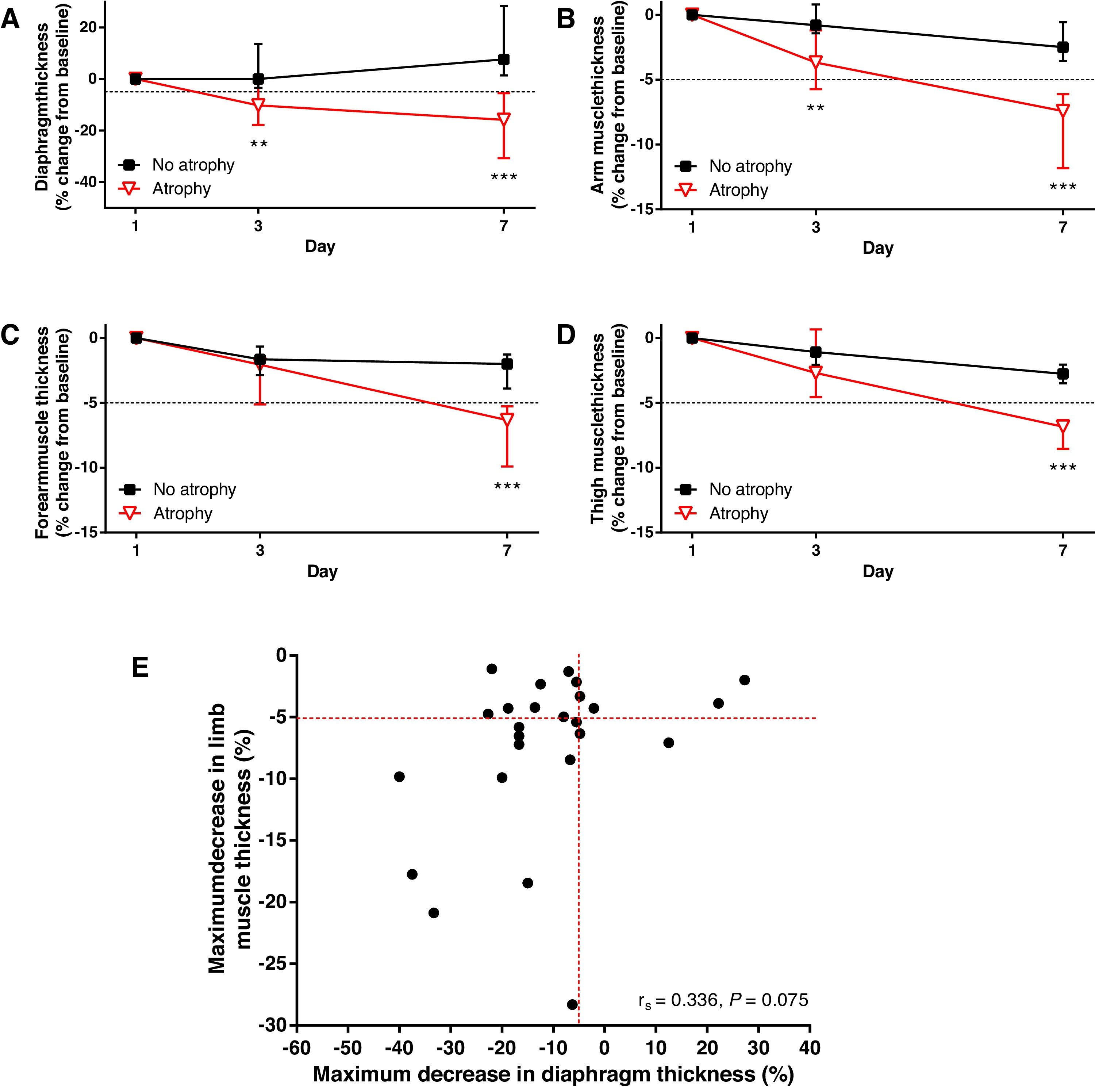
Patients’ characteristics are summarized in Supplementary Table 1. Median time on mechanical ventilation was 11 (5, 13) days and ICU mortality was 25%. During the first week, patients were mostly ventilated in assisted/controlled modes. All 24 patients that survived after ICU admission were weaned from mechanical ventilation (20 extubated, 4 tracheostomized after the first week) and discharged alive from the hospital. Mean baseline diaphragm thickness on admission was 1.7±0.5mm. This measurement was slightly higher in men versus women (1.9±0.4mm vs. 1.5±0.6mm, P=0.066), but not statistically significant. Diaphragm thickness evolution was explored in 29 subjects. Twenty (69%) subjects developed diaphragm atrophy during the first week after ICU admission. In this group of subjects, diaphragm thickness change from baseline was −10.3% (−17.8%, 0.0%) on day 3 and −15.8% (−30.7%, −5.5%) on day 7 (P=0.010 and P=0.003 compared with subjects without diaphragm atrophy, respectively; Fig. 1A).
(A) Change in diaphragm thickness over time in subjects with and without diaphragmatic atrophy. (B–D) Change in mid-upper arm (B), mid-forearm (C) and mid-thigh (D) muscle thickness over time in subjects with and without limb muscle atrophy. (E) Relationship between maximum decrease in diaphragm and limb muscle thickness.
Dashed lines represent the cutoff change in muscle thickness to define atrophy (−5% from baseline). Symbols and bars in A–D represent median and 25th–75th percentiles. **=P≤0.01, ***=P<0.001, between groups at selected time points. rs=Spearman's correlation coefficient.
Mean arm, forearm and thigh baseline muscle thickness were 2.49±0.62cm, 3.24±0.53cm and 3.01±0.54cm, respectively. Overall, men presented higher peripheral muscle thickness than women on admission (arm: 2.60±0.68cm vs. 2.20±0.33cm, P=0.033; forearm: 3.34±0.55cm vs. 2.97±0.38cm, P=0.079; thigh: 3.19±0.45cm vs. 2.56±0.51cm, P=0.002). Evolution of arm muscle thickness was explored in 31 subjects, 12 (38.7%) of whom developed atrophy. Evolution of forearm and thigh muscle thickness could be measured in 29 subjects. Forearm atrophy was observed in 11 (37.9%) subjects, while 10 (34.5%) subjects acquired quadriceps atrophy. The change in limb muscles thickness was significantly different among patients with or without atrophy (Fig. 1B–D). When considering the evolution of all three peripheral sites, 19 (61.3%) patients developed limb muscle atrophy affecting at least one of the explored muscles.
Thirteen (44.8%) subjects presented an association of diaphragmatic and limb muscle atrophy (Fig. 1E). The combination of respiratory and limb muscle atrophy was the most common profile (44.8%), as compared to patients presenting only diaphragmatic atrophy (20.7%), only peripheral muscle atrophy (13.8%) or no atrophy at all (20.7%). No significant correlation was found between the maximum decrease in diaphragm and limb muscle thickness (rs=0.336, P=0.075).
Demographic, clinical or therapeutic characteristics and baseline muscle thickness were similar among patients who developed atrophy and those who did not (Supplementary Tables 2–4 and Supplementary Fig. 2).
Baseline thickness of all the explored muscles was also similar between survivors and non-survivors (Supplementary Fig. 3). Duration of mechanical ventilation, ICU and hospital length of stay was not significantly different between subjects with or without diaphragmatic, limb or combined muscle atrophy (Supplementary Tables 2–4). Although there was a trend towards increased mortality in subjects who developed diaphragm atrophy and those who developed limb muscle atrophy compared to those who did not, these differences did not reach statistical significance. On the contrary, subjects associating diaphragm and limb muscle atrophy had a significantly higher ICU mortality than the rest (38.5% vs. 6.3%, P=0.047, Supplementary Table 4).
Being a pilot study, the principal limitations are related to the reduced sample size. Therefore, results must be interpreted with caution until confirmed in further studies involving a greater number of patients. Also, muscle function was not assessed and data regarding nutritional support was not obtained.
In conclusion, distinct atrophy profiles can be identified in critically ill patients. While muscle mass surrogates measured upon ICU admission may provide information related to the patient previous condition, their evolutionary pattern probably reflects a combination of the patient's premorbid status and concurrent critical disease and treatment. Therefore, serial muscular evaluation could be of greater importance than a single measurement at any time point. To the best of our knowledge, this is the first report of a cumulative impact of diaphragm and limb muscle wasting on ICU mortality.
Authors’ contributionsMA designed the study; AC enrolled the subjects and performed the ultrasounds; AC, SP, MB and MA contributed substantially to the data analysis and interpretation; AC and MA wrote the manuscript.
FundingNone.
Conflicts of interestNone.