We report the case of a 2-year-old female with chronic suppurative lung disease associated with esophageal candidiasis revealing a rare, recently described genetic disease: heterozygous STAT1 gain-of-function mutation.
E., a 2-year-old female, was referred to a pediatric pulmonologist for persistent wet cough. No past family records and no inbreeding are to be found. In the first six months, the infant was constantly crying and suffered from anorexia. Esophagitis was suspected, and she was treated – unsuccessfully – with esomeprazole 1mg/kg. Then cow milk allergy was suspected, and no cow milk protein milk was given for two months with no clinical benefits. At 11 months failure to thrive was diagnosed.
Chronic wet cough began at 6 months. She had recurrent rhino pharyngitis and acute otitis media. She had twice left inferior pneumonia. She also had aphthae, and persistent mouth candidiasis, despite prolonged adequate treatment: oral miconazole for 2 months, followed by 3 weeks of amphotericin B.
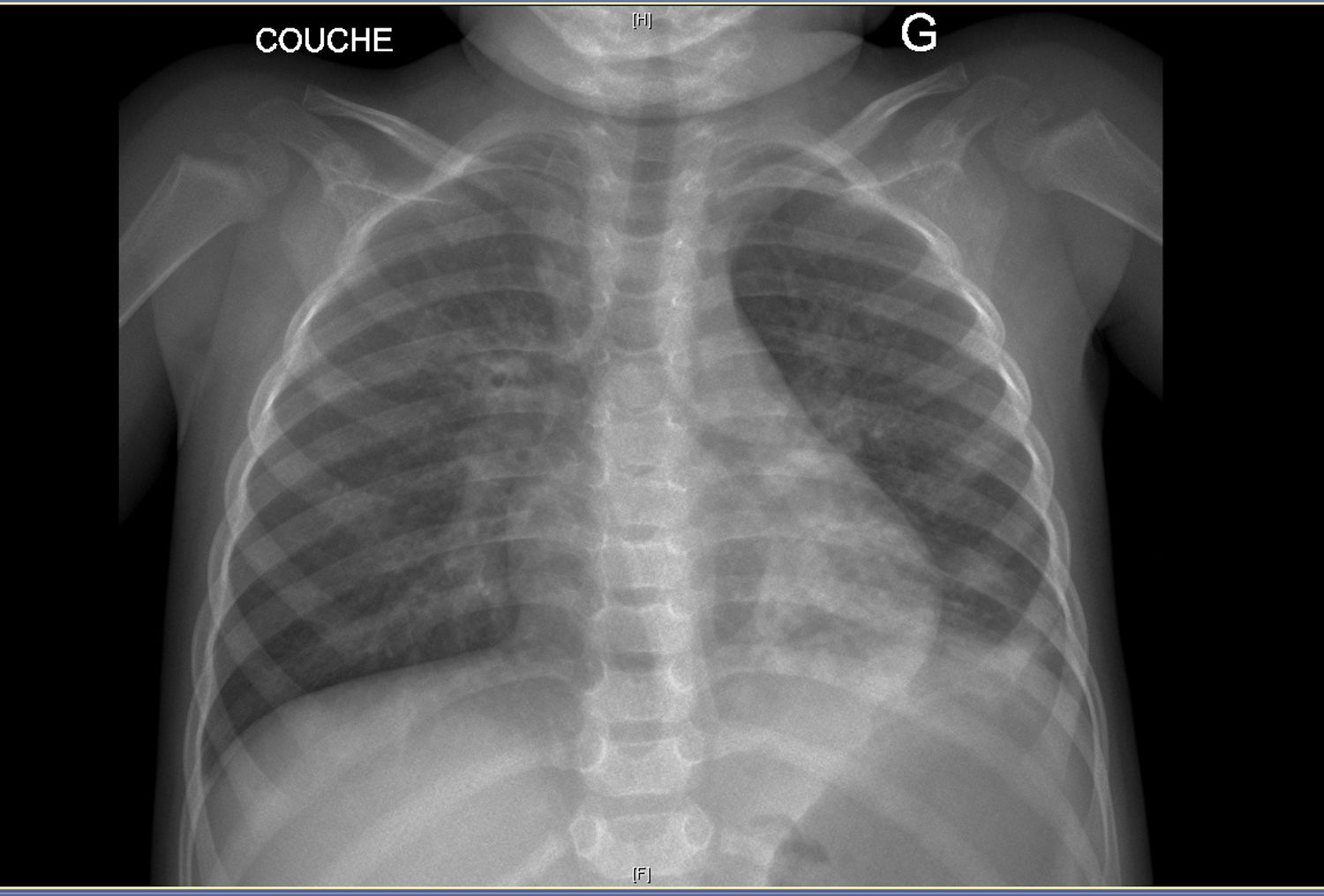
On clinical examination, she was eupneic (breath rate 27/min) with 100% oxygen saturation. She had no chest deformity, pulmonary auscultation found crackles in the left lower lobe. Fold candidiasis was visible. Chest radiograph (Fig. 1) showed left inferior opacity, consistent with partial atelectasis, associated with bilateral bronchial thickening. CT scan (Fig. 1) confirmed partial atelectasis of the lingula, medial segment of the middle lobe, anterobasal segment of the left lower lobe and small bronchiectasis of the anterior segment of the left upper lobe. Fiberoptic bronchoscopy showed thick, diffuse secretions, predominantly in the left bronchial tree (lingula); bronchoalveolar lavage was inflammatory (79% of neutrophils) with 108UFC/ml Haemophilus influenzae. Gastro-esophageal endoscopy revealed erythematous esophagitis with white deposits, consistent with candidiasis (confirmed by biopsy). The main etiologies of chronic lung suppurative disease were ruled out:
- -
sweat test (sweat chloride=20mmol/l N<60),
- -
immunological test: complete blood count (white cells 6280/mm3; hemoglobin=11.1g/dl; platelet=458,000/mm3); immunological quantitation: IgG 15.8g/dl (3.35–8.96); IgM=1.15g/l (0.48–1.53); IgA=1.08g/l (0.27–1.22); immunoglobulin G1=10.380g/l; IgG2=1.91g/l; IgG3=1.69g/l; IgG4=0.01g/l; tetanus serology=0.37UI/ml (N>0.1), pneumococcus serology=43.9mg/l; total IgE <2Ku/l,
- -
barium swallow and pH readings were normal.
She was initially treated with 7 days of intravenous ceftriaxone, and fluconazole (14 days), chest physiotherapy, and 3% hypertonic saline nebulization. Because of the association of bronchiectasis and persistent candidiasis, she was referred to the pediatric immunologist, who completed immunological tests:
- -
lymphocytic phenotyping showed mild T CD4− CD8 and Natural Killer lymphopenia, with normal count of B lymphocytes (CD4+ 546/μl; CD8+=258/μl; CD19+ 576/μl; CD 16+56+=76/μl).
Because, Mendelian predisposition to fungal infections was suspected, DNA sequencing of STAT1 (NM_007315.3) gene exons was done: it identified a heterozygous mutation C.1154C>T (p.T385M) in exon 14 of STAT1 gene. This mutation is listed among immunodeficiencies, and is responsible for predisposition to fungal infections. It is responsible of a STAT1 gain-of-function mutation. This was a de novo mutation, as it was not found in any of the two parents.
On follow-up she presented monthly exacerbations, with streptococcus pneumoniae and haemophilus influenzae on expectoration, treated with oral amoxicillin 100mg/kg/d for 15 days. She is currently under monthly intravenous immunoglobulin (0.8g/kg) perfusions, oral daily sulfamethoxazole–trimethoprim (20mg/kg/d) and fluconazole (6mg/kg/d) prophylaxis, associated with chest physiotherapy twice weekly.
Heterozygous STAT1 gain-of-function mutation was first described in 2011. Since then, more than 50 mutations have been described. It is responsible for more than 50% of chronic mucocutaneous candidiasis (CMC). STAT1 (signal transducer and activator of transcription 1) is a protein of the JAK–STAT signaling pathway. STAT1 GOF mutations lead to accumulation of phosphorylated STAT1 in the cytoplasm and nucleus, increase the transcription of genes that promote Th1 pathway, and dysregulates Th1/Th17 balance. Because IL17 is so important for antifungal immunity on epithelial surfaces, CMC appears as a result. Several case reports have been published1–4 but Toubiana et al. published the largest case series (274 patients).5 Symptoms started around 12 months and were heterogenous. 98% of cases had chronic mucocutaneous candidiasis, with 30% of resistance to antifungal treatment, that can evolve into dysphagia and esophageal cancer. Infections are frequent whether bacterial (in 74% of cases skin and airways; with mainly staphylococcus aureus and pseudomonas aeruginosa), viral (in 38% of cases) or mycobacterial (in 6% of cases). They touch the respiratory tract in more than 50% of cases with a possibility of fungal infection. 47% of patients had recurrent bacterial pneumonia, and bronchiectasis was diagnosed in 20% of cases. Autoimmunity can be found in 40% of cases. Morbidity is severe, and mortality is premature (cancer, cerebral aneurysm). Treatment is symptomatic (polyvalent immunoglobulins, antibiotic and antifungal prophylaxis). Indications of treatment with ruxolitinib (a JAK inhibitor) are not yet defined.5
GOF STAT1 gain-of-function mutation has already been described, and pediatric immunologists are aware of it, in cases of chronic mucocutaneous candidiasis. It is not a well-known etiology of bronchiectasis or chronic suppurative lung disease among pediatric pulmonologist.
This case highlights the fact that in front of idiopathic bronchiectasis with normal basic immunological tests, in cases of associated candidiasis, DNA sequencing of exons of the STAT1 gene should be performed and/or the child referred to a pediatric immunologist.