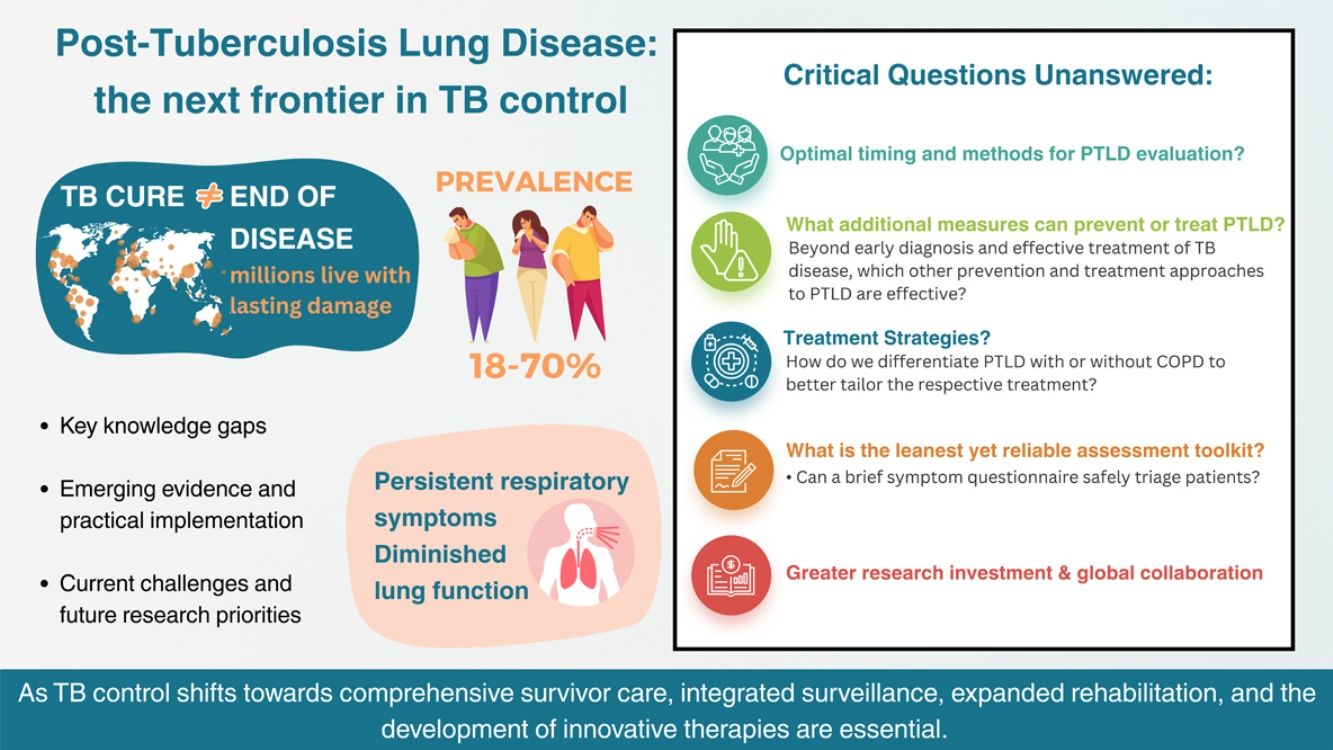
Post-Tuberculosis Lung Disease (PTLD), a persistent sequelae of Tuberculosis (TB) in which TB survivors continue to experience respiratory symptoms and diminished lung function after microbiological cure, poses a significant and growing public health challenge. Its prevalence varies widely due to differences in treatment adequacy, comorbidities, and environmental exposures and afflicts particularly high TB-burden countries. Recent advances in fundamental science, clinical investigations and exploratory trials have expanded our understanding of PTLD pathogenesis and progression. Yet, critical questions remain unanswered: Which patients are most at risk? How can we intervene early to prevent or mitigate disability? And how should healthcare systems adapt to monitor, manage, and support the growing population of TB survivors?
This review aims to inform ongoing efforts in clinical care, research, and policy. We summarize recent evidence on PTLD—specifically disease mechanisms, clinical manifestations, diagnostic approaches, risk factors, and treatment strategies. It also highlights key knowledge gaps and implementation challenges and proposes research priorities to steer future inquiry and practice. Greater research investment and stronger global collaboration are needed to mitigate the long-term burden of PTLD and improve outcomes for TB survivors.
Despite afflicting humans for more than a millennia, tuberculosis (TB) remains the world's leading infectious disease killer, claiming the lives of nearly 1.1 million people globally in 2023 [1]. Intensified global efforts, particularly through World Health Organization (WHO)-led initiatives, have markedly improved treatment coverage and cure rates, yet, TB continues to impart significant chronic morbidity to survivors [2]. It is estimated that over 155 million individuals worldwide are TB survivors, but microbiological cure does not mark the end of their illness [3,4].
Definition and epidemiology of Post-Tuberculosis Lung DiseasePost-Tuberculosis Lung Disease (PTLD) is defined by the 2019 consensus from First International Symposium on Post-TB Lung Health, as “evidence of chronic respiratory abnormality, with or without symptoms attributable at least in part to previous pulmonary TB” [5]. While this consensus definition has since been widely adopted, it remains general in scope and insufficiently specific, underscoring the necessity for refinement and a framework to classify severity. Epidemiological evidence indicates that PTLD affects nearly half of TB survivors, though prevalence varies significantly by geography, TB treatment adequacy, and population characteristics [6–8]. For instance, a 2025 Australian cohort, reported a 68% prevalence, primarily manifesting as obstructive or restrictive deficits accompanied by fibrosis or bronchiectasis on imaging [9]. By contrast, a South African retrospective cohort reported only 18% lung impairment at an average 4.6 years post-TB diagnosis, while a Tanzanian study found 74% impairment, albeit assessed at just 20 weeks of treatment [10,11]. These persistent impairments significantly diminish quality of life and drive excess mortality among TB survivors [12–14].
Global burden of PTLDPTLD remains a significant clinical and public health challenge, particularly in high TB-burden countries [15,16]. Emerging data indicate that up to 87% of survivors experience persistent sequelae, primarily due to irreversible pulmonary damage sustained during TB disease [17]. Modelling studies suggest that globally, post-TB sequelae accounts for up to half of the total TB-related disability-adjusted life years (DALYs) [18]. Economically, PTLD imposes significant burdens through increased healthcare utilization, frequent hospitalizations, and decreased productivity [14]. Such financial strain is evident in a study across five Brazilian cities, where average monthly post-diagnosis costs reached R$4161.86 (∼USD $800), consuming up to 40% of annual income among the extremely poor [19]. A 2024 systematic review echoed this, reporting a weighted mean post-diagnostic cost of USD $680 across studies [20]. These data call for broader global post-TB care investment, even in low-incidence countries facing rising TB rates among migrants and growing survivors needs.
Increasing awareness of chronic sequelae following TB treatment has accelerated PTLD research [14]. Yet, translating findings into clinical and TB programmatic practice remains challenging due to the high degree of variability and inconsistency in existing data. This review summarizes emerging data on PTLD risk factors, disease mechanisms, clinical manifestations, diagnostic approaches and treatment options while highlighting key barriers and future research priorities. Beyond microbiological cure, a shift towards the long-term recovery is needed to foster a more holistic, patient-centred TB management.
Immunopathological mechanismsIn PTLD, the immunopathology underlying the lung damage commence during TB disease. It involves the complex interplay between the host, Mycobacterium tuberculosis (Mtb) and the environment. It comprised two components: TB disease and post-TB (i.e. after microbiological cure). In brief, it likely involves two major processes: (1) Mtb infection-driven tissue damage, and (2) pathological tissue remodelling following TB disease [21]. This review focuses on the latter i.e. post-TB. However, both components are intricately linked. Dysregulated immune responses to Mtb are believed to be the main driving force behind pathological lung remodelling seen in PTLD [22,23]. Existing evidence on immunopathology of PTLD after TB cure is limited. Which in turn limit evidence-based treatment and management of PTLD [24].
Chronic immune dysregulationHost inflammation is triggered by the invading microbe and in a healthy immune response, a balancing act between pro- and anti-inflammatory cytokines ensures clearance of the microbe with resolution of inflammation returning the immune system back to homeostasis [25,26]. In TB disease, chronic inflammation occurs with dysregulated pro-inflammatory response involving cytokines such as tumour necrosis factor-α (TNF-α), interleukin 1 β (IL1β), interleukin 12 (IL-12) & interleukin 6 (IL-6) leading to cell necrosis, granuloma formation and lung remodelling [17,23,27]. Mtb itself may skew host inflammation towards pro-inflammation that favours cavity formation and onwards transmission of Mtb for its survival benefit [28].
PTLD likely involves persistent inflammation and inability to restore homeostasis even after Mtb clearance[29]. However, residual Mtb antigens may sustain chronic inflammation. A PET-CT (Positron Emission Tomography-Computed Tomography) study reported the formation of new lesions and ongoing inflammation in lung lesions of patients 6 months post- treatment, with detection of Mtb mRNA suggesting viable but non-culturable bacilli that can elicit a chronic immune response [30]. A clinical case study also reported inflammatory post-TB lesion but with no viable Mtb[31]. Together, these findings suggest ongoing inflammation contribute to persisting lung damage in PTLD, though a role for Mtb or its antigen cannot be excluded.
Lung remodellingIn pulmonary TB (PTB), lung remodelling involves tissue destruction, caused by proteases such as host's matrix metalloproteinases (MMPs) leading to cavitation, and aberrant wound healing, with fibrosis driven by profibrotic cytokines such as transforming growth factor-β (TGF-β) and IL-6 through excessive extracellular matrix deposition [24,32–34]. The three most common residual lung damage namely fibrosis, cavities and bronchiectasis are believed to occur during PTB but remain unresolved after TB treatment [6,35]. Between 20 and 50% of TB cavities persisted after TB treatment completion with healing taking place post-TB treatment. Cavity resolution can either be complete i.e. closed with scar or calcified foci or incomplete with the cavity airspace remaining. Incomplete resolution with fibrotic scarring increases the risk of respiratory infection such as fungal infection but the mechanism of cavity closure remains unknown [17,28]. Residual cavity resolution in post-TB treatment may contribute to formation of fibrosis and abnormal dilation of airways leading to bronchiectasis. Clinical evidence of development of bronchiectasis after post-TB supports this postulation [36].
A small study of 30 PTB patients showed a lesser magnitude of decline in concentrations of profibrotic cytokine, TGF-β and IL-6 associated with greater lung impairment in PTB after TB treatment completion [37]. This potentially supports ongoing fibrosis with new fibrotic lesions post-TB which may also result from cavity resolution [17,28].
A study reported elevated plasma S100A8, MMP-1, MMP-9 and IL-8 in patients with poor lung recovery post-TB suggesting neutrophil activity and persistent leucocytes infiltration contributed to unresolved lung damage [38]. MMPs are capable of degrading extracellular matrix which makes up the structural lung tissues and this potentially drives the persistent residual damage in PTLD [33]. The immunopathology underlying the unresolved lung damage is unclear, but potentially ongoing lung remodelling involves leucocytes, lung tissue destruction and fibrosis. Further investigation is warranted to address these gaps.
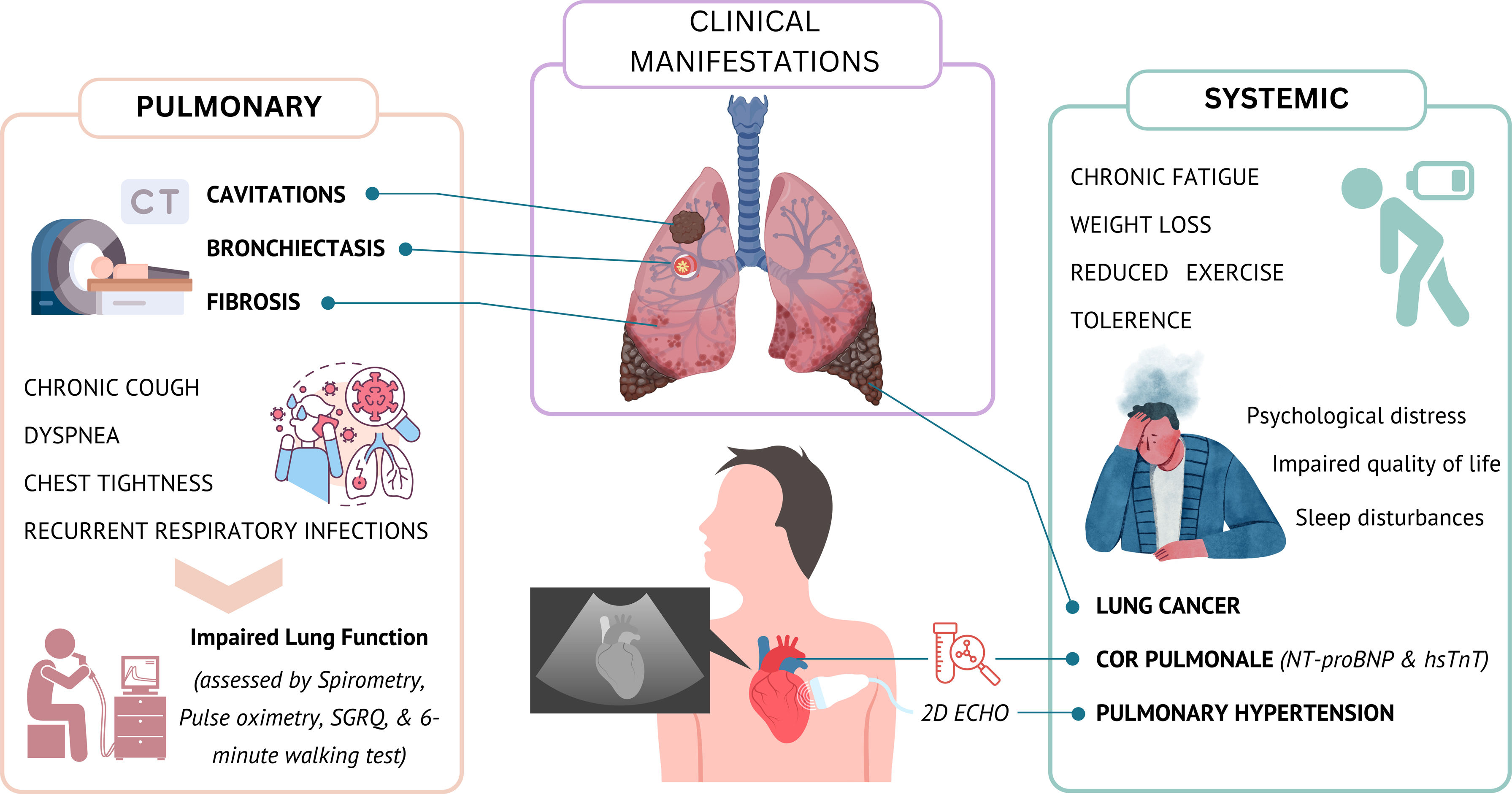
Clinical manifestations and diagnosisDue to the complex and heterogenous nature of PTLD, it cannot be characterized by a single defining manifestation or diagnosed by a single test. Patients with PTLD may present with a wide range of symptoms including chronic cough, dyspnoea, chest tightness, recurrent respiratory infections, impaired pulmonary function, as evidenced by reduced forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) on spirometry [39]. In more severe cases, hemoptysis may occur due to bronchiectasis or residual cavitary lesions [35,40]. Beyond pulmonary symptoms, systemic effects include reduced exercise tolerance, chronic fatigue, weight loss, and psychological distress [14,41] (Fig. 1). Evaluation should therefore be comprehensive, integrating clinical, functional, and radiological assessment. Recent studies also suggest higher cardiovascular morbidity driven by pulmonary vascular remodelling, notably pulmonary hypertension and right heart failure (cor pulmonale) secondary to chronic hypoxemia [12,42,43]. Lung cancer further contributes to this burden [44].
Clinical manifestations of PTLD: Visual summary of common pulmonary lesions and symptoms (left) versus extra-pulmonary manifestations (right) observed after TB treatment. CT=computed tomography; SGRQ=St. George's respiratory questionnaire; NT-proBNP=N-terminal pro-brain natriuretic peptide; hsTnT=high-sensitivity troponin T; 2D Echo=two-dimensional echocardiogram.
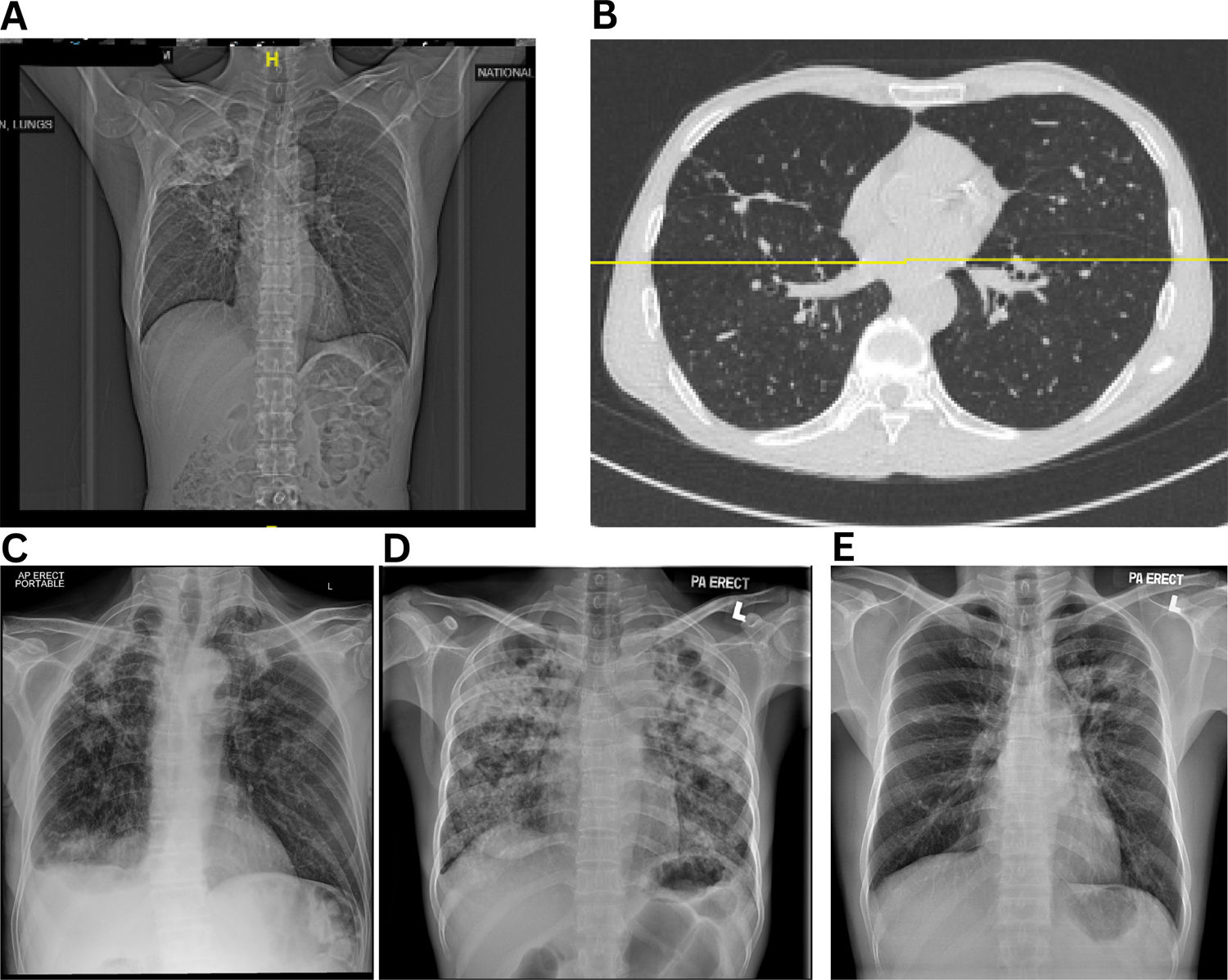
Most studies on PTLD risk are cross-sectional or retrospective, limiting causal inference or identifying early predictors [7]. The absence of simple, scalable diagnostic tools for resource-limited settings further compounds the challenge. Although some studies followed up beyond 6–9 months post-treatment, most focused primarily on spirometry, offering limited insights into PTLD's natural history, and progression [39,40,45]. Imaging remains central to diagnosis: chest radiographs may reveal residual fibrotic bands with volume loss, bronchiectasis, pleural thickening, cavities, or calcifications [46] (Fig. 2), while high-resolution computed tomography (HRCT) provides more detailed characterization [36]. Nevertheless, radiologic severity often poorly correlates with symptoms or functional decline, complicating clinical interpretation [47].
Representative radiographic and high-resolution CT images of patients with microbiologically confirmed pulmonary TB. (A) Extensive right upper lobe volume loss, fibrotic scarring, and tracheal deviation to the right—hallmarks of post-tuberculosis fibrotic lung damage, (B) Bilateral bronchiectasis with dilated bronchi, parenchymal scarring, (C) Cavitary lesions and right upper lobe fibrosis, (D) and (E) Severe fibrosis and bronchiectasis.
Pulmonary function testing complement radiology, often revealing a mixed restrictive/obstructive patterns with predominant airflow obstruction [45]. Yet, spirometry access remains limited in many high TB-burden settings, and longitudinal follow-up beyond lung function impairment assessment is lacking [39]. Moving forward, prospective studies assessing the full spectrum of PTLD and consensus-based diagnostic algorithms are essential. Integration of spirometry and symptom screening, coupled with validated imaging scores into routine post-treatment care, would enable earlier detection and intervention.
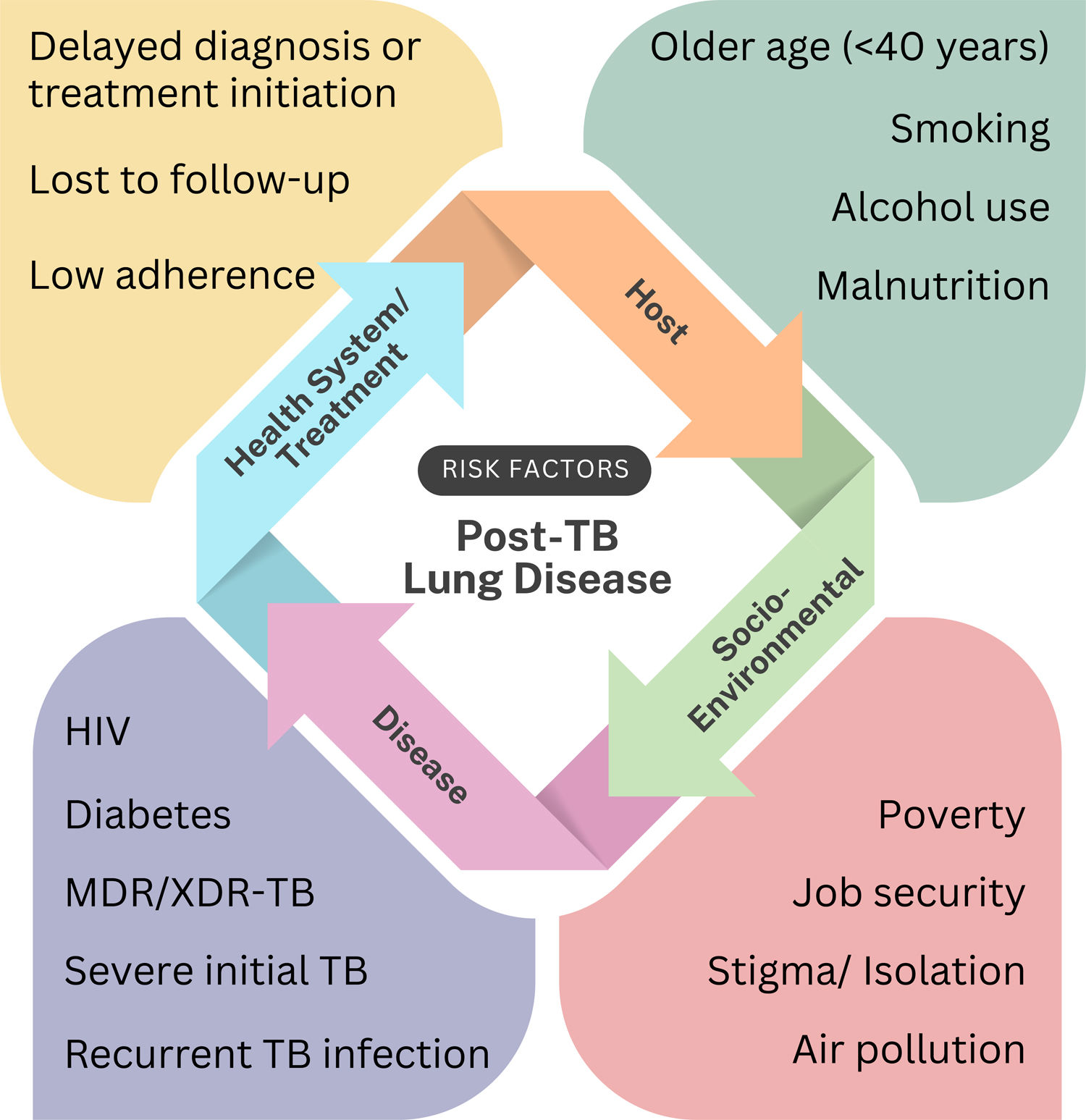
Risk factors and prognosisSeveral clinical characteristics and comorbidities are linked to long-term lung damage and functional impairment in TB survivors [40] (Fig. 3). However, most evidence stems from small-scale or cross-sectional studies, with limited robust epidemiological data and longitudinal cohorts tracking progression of PTLD from TB treatment [24]. This hampers accurate prediction of disease trajectory and reliable prognostic markers essential to guide targeted intervention strategies.
A recent meta-analysis identified extensive lung lesions at diagnosis (often due to late care-seeking or poor access to healthcare), age>40, smoking, malnutrition, HIV and diabetes as key risk factors for post-TB lung sequelae [48]. Elevated cytokines (e.g., IL-6, TNF-α) and MMPs (MMP-1, MMP-9) are associated with poorer TB treatment outcomes, and not directly to PTLD [49–51]. However, since poorer TB treatment outcomes lead to prolong infection, increased lung injury and fibrosis, these biomarkers may also represent risk factors for PTLD. Identifying robust prognostic markers is critical for risk stratification and personalized interventions, and reducing chronic morbidity.
Given asthma and Chronic Obstructive Pulmonary Disease (COPD) frequently coexist with TB in endemic regions, future research must recruit well characterized PTLD cohorts – irrespective of comorbidities to identify PTLD-specific risk factors, rather than rely on data extrapolated from other airway diseases. Refining methods to detect PTLD early in low-income, high incidence regions will facilitate cost-effective prevention and mitigate lifelong respiratory impairment.
Current treatment approachesPharmacological & non-pharmacological therapiesA key approach is the prevention of TB disease at the outset. Prevention would reduce the risk of PTLD and mitigate PTLD signs and symptoms and progression [35,40,46]. Furthermore, other respiratory co-morbidities such as infections in residual cavities including Aspergillus and non-tuberculosis mycobacteria, bronchiectasis and lung cancer and non-respiratory co-morbidities would be prevented [52–55]. Key in preventing TB disease is preventing individuals from being infected by M. tuberculosis and subsequent TB disease, utilizing all existing preventive measures (infection control, vaccination-ideally with new vaccines, treatment of TB infection) [40]. Rapid diagnosis and effective treatment after TB disease diagnosis are key pillars for breaking the chain of transmission, a core aim of all global TB programmes [40]. Reducing environmental risks factors, such as air pollution and tobacco exposure, also plays an important role [40].
Recent evidence suggests the important preventive role of vaccinations in adults and children [5,46,56–59]. On completion of anti-TB treatment, children should be ensured to be updated in vaccinations as per national immunization schedule specifically tetanus, diphtheria, pertussis and measles [58]. In adults, vaccination against influenza, pneumococcal disease, and COVID-19 can be of help to avoid exacerbations as PTLD is a chronic respiratory disease [58]. Host-directed therapies targeting pathology or bacillary clearance are described elsewhere in this review [35,60]. For PTLD, managing of co-existing conditions – asthma and COPD – is essential, often requiring inhaled steroids and bronchodilators [40,46,59].
Diagnosing and treating recurrent TB (e.g. relapses if diagnosed after established treatment success) is of obvious importance. Whenever superimposed infections occur, tailored antibiotic treatment is indicated. In selected patients, surgical interventions such as lobectomy or pleurectomy may be indicated, while bronchiectasis may benefit from anti-inflammatory treatment. Finally, availability of palliative care and end-of-life support is mandatory for patients with unfavourable prognosis. The Union's Clinical Standards highlights the importance to undergo quality health education and counselling for PTLD patients [40].
Pulmonary rehabilitation: emerging evidence and practical implementationThe Clinical Standards and other documents emphasized the importance of pulmonary rehabilitation as one of the pillars of PTLD management [15,35,40,46,59,61–65]. This panel of global expert highlighted that most of the evidence available on pulmonary rehabilitation was based on a few retrospective and uncontrolled studies with a limited sample size of 30–40 patients [40,64]. Therefore, evidence was extrapolated from COPD. A recent multicenter study in Brazil enrolled 85 PTLD patients undergoing rehabilitation and 96 controls, with one year follow-up [15,62,64]. Rehabilitated patients achieved significant improvements in spirometry (FEV1, FVC), DLCO, the distance walked at 6MWT and oxygen saturation. Although benefit was most pronounced in those with COPD, all rehabilitated PTLD had a significant improvement, while non-rehabilitated patients deteriorated. This pivotal study unfortunately did not evaluate the Quality of Life (QoL) [64].
Beyond the Union's Clinical Standards on PTLD, ad-hoc algorithms are needed at the national level to screen patients completing anti-TB regimens for PTLD. Suspected cases should be evaluated and referred for pulmonary rehabilitation when indicated. Through pre- versus post- analysis of the core variables, the effectiveness of rehabilitation will be established. Patients should receive training on maintaining improvements through home-care interventions and tailored follow-up.
Emerging research and host-directed therapiesAnimal models studying PTLD have rarely been used, as most models do not study the effects after TB treatment. Findings are often extrapolated from murine, guinea pigs, rabbits and non-human primate models of pulmonary TB. Granulomas – a hallmark of TB disease – are absent in common murine strains such as C57BL/6 and BALB/c. However, persistent inflammation and structural lung damage with fibrosis are consistent across animal models. Boucau et al. described a Kramnik mouse model that developed necrotizing granulomas with features of fibrosis 3 months into infection which did not resolve with a further 8 weeks of TB treatment and inflammatory signatures remaining weeks into treatment [66]. The inflammatory signature was associated with pro-fibrotic macrophages even in the presence of TB treatment. Navitoclax, a proapoptotic Bcl-2 inhibitor, may reduce the development of fibrosis in TB-treated Kramnik mice [67]. Another murine model that develop necrotizing granulomas is the hyper-susceptible Nos-2 mice but PTLD models of this strain have yet to be established [68]. Structural lung damage post-TB treatment has similarly been observed in guinea pigs, rabbits, and non-human primates [69–71].
Common immunomodulators such as steroids and non-steroidal anti-inflammatory agents (NSAIDs) have limited use in preventing PTLD. A Cochrane systematic review reported that steroids did not reduce all-cause mortality and had no effect on sputum culture conversion or lung function [72]. A study investigating the effects of ibuprofen in XDR-TB (NCT02781909) is awaited. Other NSAIDs such as aspirin have been investigated in central nervous system TB with notable effects on the incidence of gastrointestinal bleeding but not in the context of PTLD [73]. Overall, current evidence does not support the use of steroids or NSAIDs for PTLD.
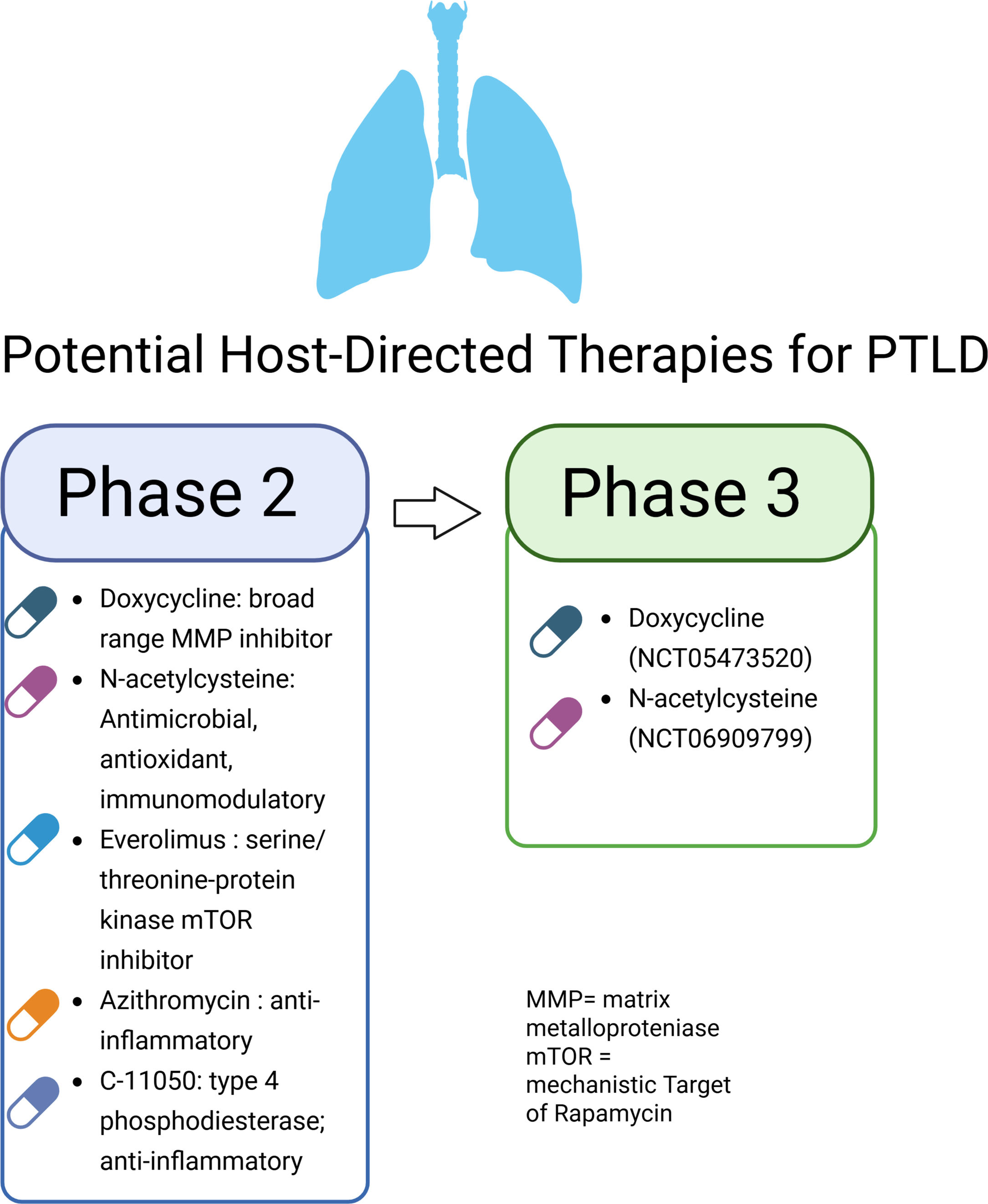
Human trials examining adjunctive drugs for PTLD are on the rise. Phase 2 trials have investigated doxycycline, everolimus, CC-11050, N-acetylcysteine (NAC) and azithromycin (Fig. 4), all found to be safe with standard TB treatment [74–77]. In a placebo-controlled, double-blinded trial, doxycycline accelerated resolution of both systemic and respiratory inflammation with reduced lung cavity volumes [74]. Separately, in an open-label trial, both everolimus and CC-11050 adjunctively treated patients showed improvement in FEV1 at 6 months post-randomization. However, both everolimus and CC-11050 can interact with standard TB drugs, specifically rifamycins. In another open-label trial, NAC treated patients showed improvement in both FVC and FEV1 [76]. Meanwhile, azithromycin adjunctively treated patients showed less neutrophilic inflammation in the respiratory compartment without clear clinical benefits. Larger Phase 3 trials of doxycycline (NCT05473520) and N-acetylcysteine (NCT06909799) are underway.
Altogether, animal models of PTLD are limited to PTB-treated animals and not post-treatment with potential agents holding promise to translation into human use. In contrast, human Phase 2 clinical trials show promise. Upcoming Phase 3 trials of affordable, widely available agents such as doxycycline and NAC hold potential use to reduce the global burden of PTLD and its associated mortality.
Challenges for the road aheadBarriers to overcomeRaising awareness of PTLD as a global health priority is essential, as it accounts for nearly half of TB-related DALYs [40]. Unfortunately, most National TB programmes (NTP) focus solely on reducing incidence and mortality, considering their work done at TB cure [1]. Limited resources and lack of understanding that PTLD is both preventable and treatable – especially in high-burden countries – hinders implementation of pulmonary rehabilitation [62,64]. The lack of evidence from priority countries further impedes progress. While rehabilitation has been shown to benefit PTLD patients, in settings with high incidence but scarce resources it may not be feasible to provide it universally. Prioritization of those most likely to benefit within locally adapted rehabilitation schemes is therefore critical. After Brazil published the first-ever national guidelines on PTLD management [46], and the Latin American Society of Pulmonology (ALAT) published its regional guidance [59], failure to offer rehabilitation when feasible has to be considered unethical. WHO has begun addressing this gap with a policy brief (Integrated approach to tuberculosis and lung health) [78], and upcoming PTLD management guidance. A coordinated global effort is needed to identify suitable, country- and setting-specific models allowing programmatic implementation.
Surveillance and long-term managementWHO revised treatment outcome definitions include ‘sustained treatment success’ to capture relapses after initial cure, recommending re-evaluation 6–12 months post-treatment [79]. However, most NTPs from high-TB burden countries lack the resources for routine follow-up. It is more feasible under special conditions, such as clinical trials, observational studies, or ongoing rehabilitation. Currently, no TB registry worldwide records rehabilitation outcomes to generate meaningful indicators. This gap could be addressed through pilot programmes in settings where PTLD rehabilitation has been implemented.
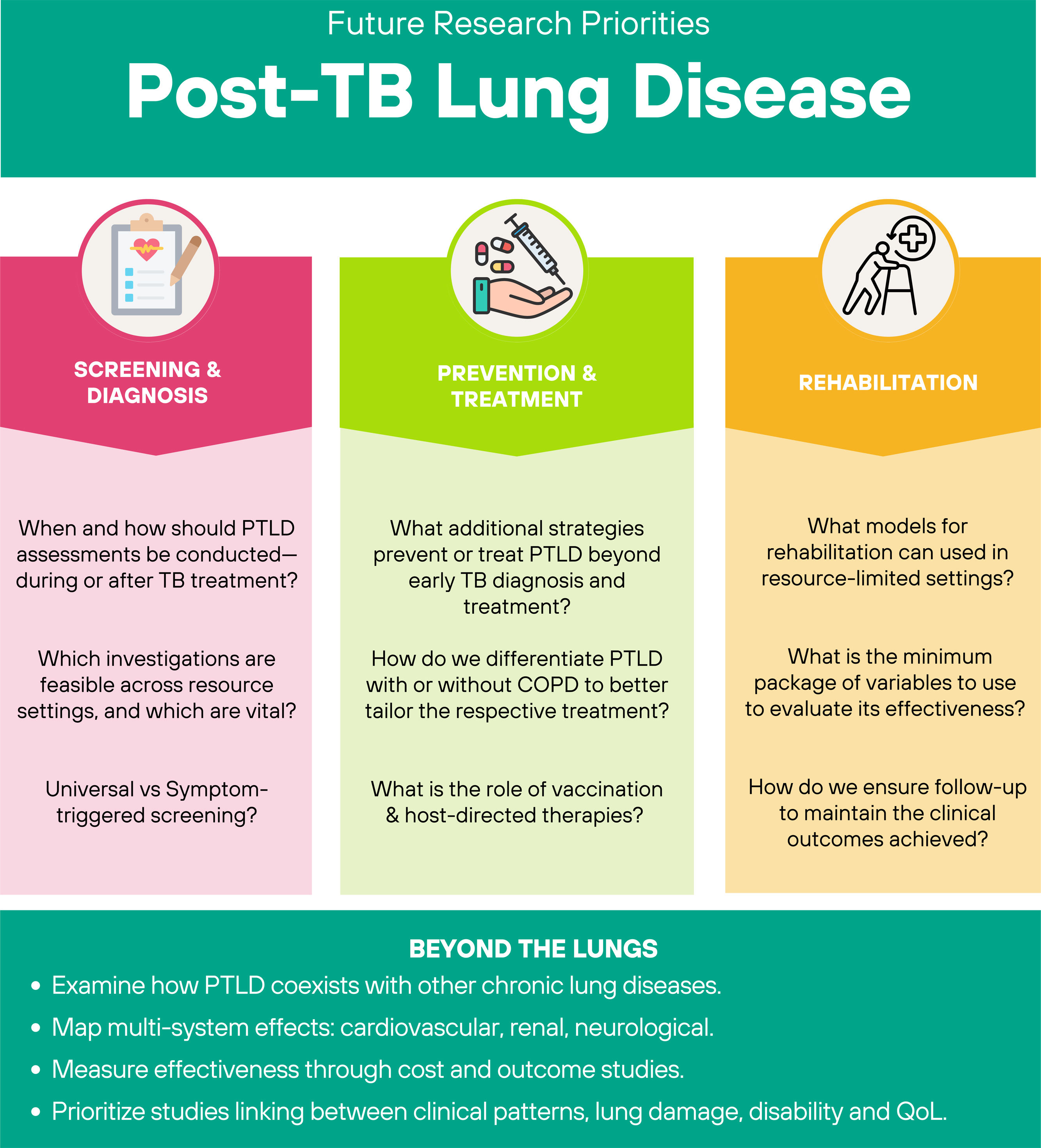
Future research prioritiesEarlier publications outlining future research priorities for PTLD, proposed approaches for screening, diagnosis, treatment and rehabilitation [5,40,56,61]. However, not all evidence was available and there was a lack of validation of a suitable approach, often extrapolated from COPD [40]. A recent meta-analysis further emphasized significant data heterogeneity, limiting evidence synthesis [6]. At the time of the Union's Clinical Standards and the proceedings of the Stellenbosch Symposia, most studies were small, retrospective, and lacked controls or follow-up [5,40,56]. Key research questions requiring urgent attention are summarized in Fig. 5.
- a)
Screening and diagnosis. How should assessments for PTLD be conducted in patients who complete TB treatment? Can assessment be done during TB treatment? Which investigations can be done across different resource settings? Are some more important than others? Should all patients be screened or only those with signs and symptoms? Can a symptom-based questionnaire reduce the number of patients to be screened for PTLD?
- b)
Prevention and treatment. Beyond early diagnosis and effective treatment of TB disease, which other prevention and treatment approaches to PTLD are effective? What is the real role of vaccination? How do we differentiate PTLD with or without COPD to better tailor the respective treatment? What is the role of host-directed therapies?
- c)
Rehabilitation. What models for rehabilitation can be used in resource-limited settings? What is the minimum number of variables to use to evaluate its effectiveness? How do we ensure follow-up to maintain the clinical outcomes achieved?
A recent Brazilian trial, which included a control group of non-rehabilitated PTLD patients, demonstrated the feasibility of prospective controlled studies to evaluate the efficacy of interventions including rehabilitation and the importance to stratify PTLD based on COPD status and severity [62,64]. The role of co-morbidities needs to be investigated, including the triangulation existing between PTLD, COPD, aspergillosis and bronchiectasis, as well as the systemic involvement (cardiovascular, renal, neurological). Beyond feasibility and efficacy, future studies should investigate effectiveness through economic analysis and unravel the relationships between clinical patterns, lung damage, disability and QoL.
ConclusionEmphasis on importance of addressing PTLD in high and low TB incidence countriesEvidence gaps need to be tackled through existing clinical experiences across different continents before there is capacity to manage PTLD on a TB programmatic basis. In Latin America Brazil was pivotal in issuing its national programmatic guidelines [46]; which enabled the first national controlled study on the effectiveness of rehabilitation [62,64]. In parallel, ALAT has created a framework guidance allowing all NTPs to develop own national guidelines [59]. Furthermore, ALAT is presently developing the region's first ever PTLD registry to document prevalence, gauge disease severity, and test the feasibility and efficacy of its rehabilitation.
In Mexico outreach activities to diagnose and manage PTLD started in spring 2025 building on an existing pilot COPD project. In Bangladesh, Damien Foundation's TB REACH project, is implementing PTLD management in four rural districts: several hundred patients have already been screened, and dozens are undergoing pulmonary rehabilitation [15]. The comprehensive project – covering prevention, screening, treatment support, and referral rehabilitation – integrates seamlessly with active case-finding, TB infection management, and smoking-cessation services. This game-changing model demonstrates how single studies or pilot experiences can be scaled into programmatic public health interventions, taking into account the present funding difficulties in the USA [80].
Call for collaborative research and innovationThe difficulties to implement complex interventions like those necessary to manage PTLD, which include screening, diagnosis, prevention, treatment and rehabilitation, are well known. A rational approach to PTLD is particularly urgent in resource-limited settings, where TB and PTLD are more prevalent [52,53]. Ongoing trials of host-directed therapies like doxycycline and NAC may expand treatment options. Clinicians, researchers and patients are called to collaborate with scientific societies and journals in order to share best practices and advocate for funding, more research and quality clinical services to alleviate the significant burden PTLD patients face.
Authors’ contributionAll authors have contributed equally to the preparation and review of this article.
Artificial intelligence involvementDuring the preparation of this work, the authors used ChatGPT-4 to assist with condensing the text to meet the journal's word limit. All content was subsequently reviewed by the authors, who take full responsibility for the content of this publication.
Funding statementC.W.M.O. is funded by Singapore National Medical Research Council (CSASI24jul-0005), the Infectious Diseases Translational Research Programme in NUSMedicine, and iHealthtech at the National University of Singapore.
Conflicts of interestsThe authors declare no competing interests.