The aim of this study is to evaluate changes in the risk of cardiorespiratory mortality and morbidity calculated by Eurolung risk models 1 and 2 in the last 20 years, and to identify variations in patient selection or surgical practice that might have altered the risk of death and complications after anatomical lung resections.
MethodThis was a retrospective analysis of a series of 2435 consecutive patients who underwent anatomical lung resection. The population was divided into three time periods: 1994–2006 (976 cases), 2007–2015 (945 cases), and 2016–2017 (420 cases). Eurolung models 1 and 2 were applied to the series, and the individual probability of adverse effects was calculated. We compared this mean probability, and the prevalence or means of each of the variables included in the models in each period and plotted the evolution of the risk.
ResultsA progressive decrease was observed in both adverse effects over time. The prevalence of the binary variables, except for coronary heart disease, was higher in the last period. The percentage of pneumonectomies and extended resections fell in the last two periods and the number of cases treated with VATS increased substantially in 2016–2017.
ConclusionsThe decline in the number of pneumonectomies and the increase in the rate of minimally invasive procedures appear to be the variables most closely associated with decreased risk. Other changes in the clinical characteristics of the patients do not seem to have influenced the outcomes.
El objetivo del estudio es evaluar las modificaciones del riesgo de mortalidad y morbilidad cardiorrespiratoria calculada mediante los modelos de riesgo Eurolung 1 y 2 en los últimos 20 años para identificar variaciones en la selección de los pacientes o en la práctica quirúrgica que hayan conducido a cambios en el riesgo de muerte y complicaciones tras resecciones anatómicas pulmonares.
MétodoAnálisis retrospectivo de una serie de 2435 casos consecutivos sometidos a resección pulmonar anatómica. La población fue dividida en tres períodos de tiempo: 1994-2006 (976 casos), 2007-2015 (945 casos) y 2016-2017 (420 casos). Se aplicaron los modelos Eurolung 1 y 2 a la serie y se calculó la probabilidad individual de efectos adversos. Se comparó dicha probabilidad media, así como la prevalencia o las medias de cada una de las variables que constituyen los modelos en cada período y se representó gráficamente la evolución del riesgo.
ResultadosSe observó un descenso progresivo de ambos efectos adversos a lo largo del tiempo. La prevalencia de las variables binarias, excepto enfermedad coronaria, fue mayor en el último período. El porcentaje de neumonectomías y de resecciones ampliadas descendió en los dos últimos períodos y el número de casos abordados por VATS se incrementó considerablemente en 2016-2017.
ConclusionesEl descenso del número de neumonectomías y el incremento de la tasa de procedimientos mínimamente invasivos se consideran las variables más relacionadas con la disminución del riesgo. Otros cambios en las características clínicas de los pacientes no parecen haber influido en los resultados.
Mortality and morbidity after anatomical lung resection have fallen in recent decades, according to the annual reports of the European Society of Thoracic Surgeons (ESTS),1 with unadjusted mortality rates decreasing from 3.9% to 1.7% in the last 5 years and cardiorespiratory morbidity after lobectomy falling from 20% in 2009 to 15.2% in 2018.1
Numerous studies have estimated the perioperative morbidity and mortality and assessing predictive factors in patients undergoing lung resection. Moreover, several multivariate models have been developed in recent years with the aim of improving the selection of patients for surgery. The steering committee of the ESTS database has recently developed Eurolung 1 and 2, two robust models for calculating the adjusted risk of hospital morbidity and mortality in candidates for anatomical lung resection surgery.2 These models are based on the retrospective analysis of a population of 47960 patients included in the ESTS database who underwent surgery between July 2007 and August 2015. Eurolung 1 and 2 were created to serve as tools for quality control and for risk stratification in thoracic surgery.
Although the risk of morbidity and mortality has declined in recent years, the temporal trend in risk of death and complications based on the current ESTS models has not yet been assessed. The objective of this study was to apply the Eurolung 1 and 2 risk models to a series of patients who underwent anatomical lung resection in the last 20 years, in order to: (1) assess and compare the risk of postoperative cardiorespiratory morbidity and mortality in 3 successive time periods and (2) identify variations in the selection of patients and surgical practices that have led to changes in the risk of death and postoperative complications.
MethodPopulationThis is a retrospective analysis of a series of 2435 consecutive cases who underwent anatomical lung resection (lobectomy, segmentectomy, bilobectomy, or pneumonectomy) for lung cancer between 1994 and 2017 in our hospital. Patient data were collected prospectively in an institutional database. In order to increase the quality of the data included in the registry, the completeness and accuracy of each entry is controlled by a data manager at two different time points: first, when the patient is discharged from the hospital, and later, at the time when the final histological result is entered in the definitive medical reports. Ninety-four cases with missing data were excluded from the study, representing 3.9% of the total, so the study population included 2341 cases.
For this study, the population was divided into 3 time periods: the first period included 976 cases undergoing anatomical lung resection between 1994 and 2006 (cases prior to the construction of the current ESTS database); the second period included 945 patients undergoing surgery between 2007 and 2015 (cases that were used for the construction of the Eurolung 1 and 2 models), and the third period included 420 patients undergoing surgery between 2016 and 2017 (after the recruitment of cases for the construction of the models).
Dependent VariablesWe analyzed the risk of cardiorespiratory morbidity and mortality at 30 days calculated from Eurolung models 1 and 2 in the three study time periods.
The following equation was used to estimate the risk of morbidity (Eurolung 1): logit (morbidity): −2.465+0.497_male sex (coded 1 for men and 0 for women)+0.026_age+0.231_coronary disease (CD) (coded 1 for presence of CD)+0.371_cerebrovascular accident (CVA) (coded 1 for presence of CVA)+0.152_chronic kidney disease (CKD) (coded 1 for presence of CKD)−0.015_ppoFEV1%+0.514_extended resections (coded 1 for presence of extended resection)+0.497_thoracotomy (coded 1 for thoracotomy and 0 for VATS).
The following equation was used to estimate 30-day mortality (Eurolung 2): logit (mortality): −5.82+0.903_male sex (coded 1 for men and 0 for women)+0.044_age+0.264_coronary disease (coded 1 for presence of CD)+0.582_CVA (coded 1 for presence of CVA)−0.064_body mass index (BMI)+0.300_extended resection (coded 1 for extended resection)+0.929_pneumonectomy (coded 1 for pneumonectomy and 0 for smaller resections)+0.894_thoracotomy (coded 1 for thoracotomy 1 and 0 for VATS)−0.009_FEV1%ppo.
Independent VariablesThe same risk variables included in the Eurolung 1 and 2 models were used2: sex, age, BMI, forced expiratory volume in 1 second predicted postoperative (ppoFEV1%), CD, CVA, CKD, surgical approach (thoracotomy or video-assisted thoracoscopic surgery [VATS]), extended resection (associated with chest wall, Pancoast tumors, resection of the atrium or superior vena cava, resection of the diaphragm, vertebral resection, pleuro-pneumonectomy, pneumonectomy with tracheoplasty, intrapericardial pneumonectomy), and pneumonectomy.2
The indication for resection and final pathological diagnosis were not taken into account for study purposes.
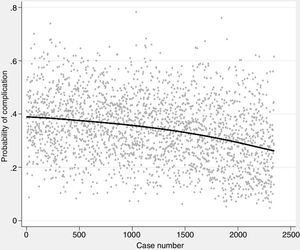
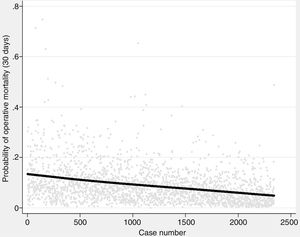
Statistical AnalysisThe individual probabilities of morbidity and mortality were maintained as new variables. We then applied the analysis of variance (ANOVA) to compare those probabilities among the three study periods and constructed box plots to represent the differences graphically. Finally, we constructed a weighted polynomial regression model (LOESS) to graphically represent the evolution of the probability of each dependent variable with respect to time.
We also calculated the frequency of different independent variables included in the Eurolung 1 and 2 models for each time period. The frequency difference of dichotomous variables (sex, cardiac or cerebrovascular comorbidity, surgical approach, extended resection, and pneumonectomy) was compared using a Chi-squared test, while the mean of the continuous variables (age, BMI, ppoFEV1%) was compared using ANOVA.
The statistical analysis was conducted using the STATA 15.1 statistical software package (Stata Corp., College Station, TX, USA).
ResultsPopulation DescriptionThe study population is composed of 2341 patients with a mean age of 65.1 years (±10.2), a ppoFEV1% of 60.1% (±20.8) and a proportion of 82.7% men. A total of 86.5% (2024) procedures were performed by lateral or posterior thoracotomy without muscle section, and 317 (13.5%) were performed by VATS. The procedures performed were: 306 pneumonectomies (of which 85 were extended and 11 were tracheoplasties), 134 bilobectomies (25 extended), 1804 lobectomies (238 extended and 84 with bronchoplastic resection), and 97 anatomical segmentectomies. Total mortality at 30 days was 56 cases (2.4%), and 371 cases (15.8%) presented 1 or more cardiorespiratory complications.
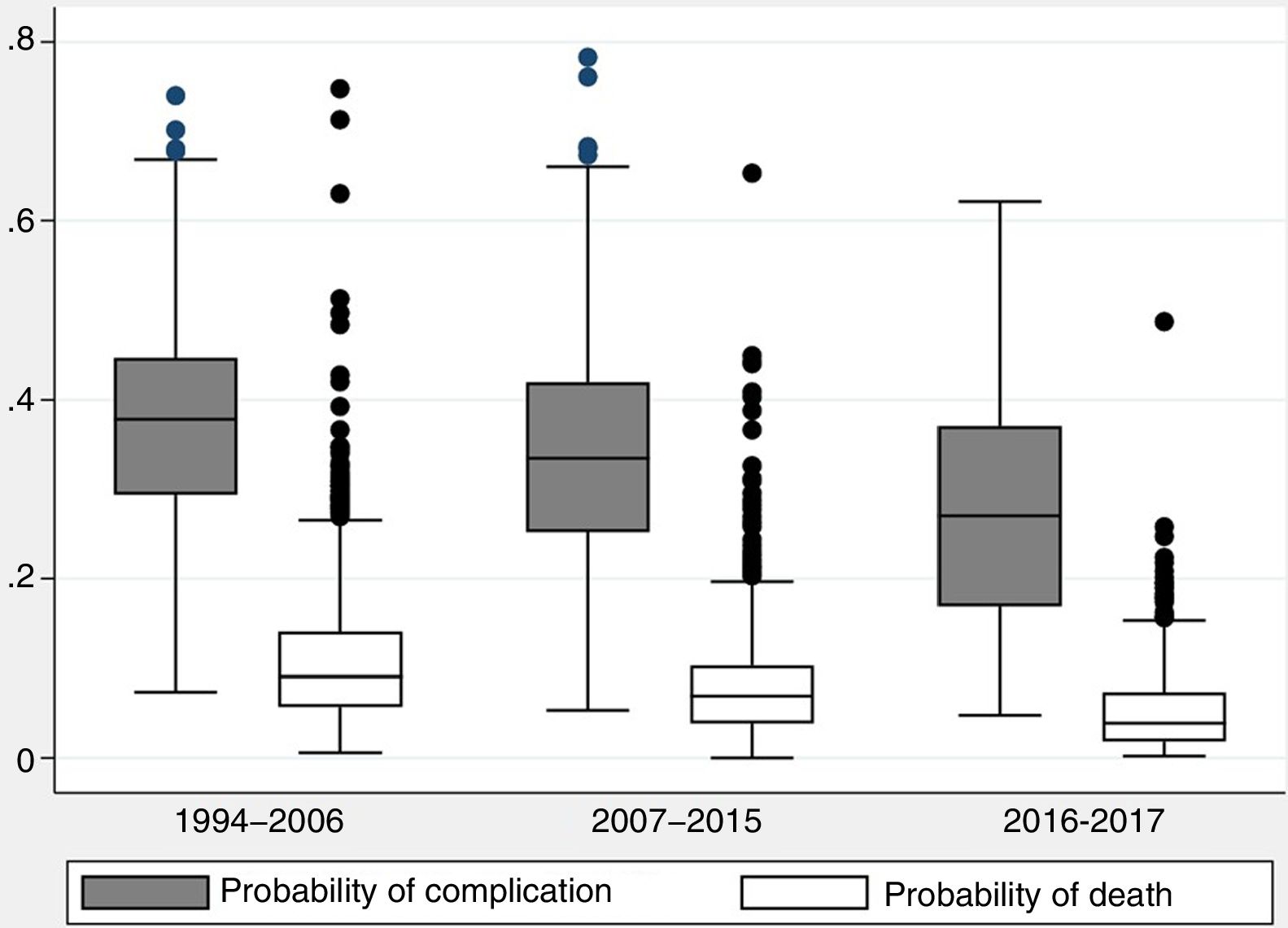
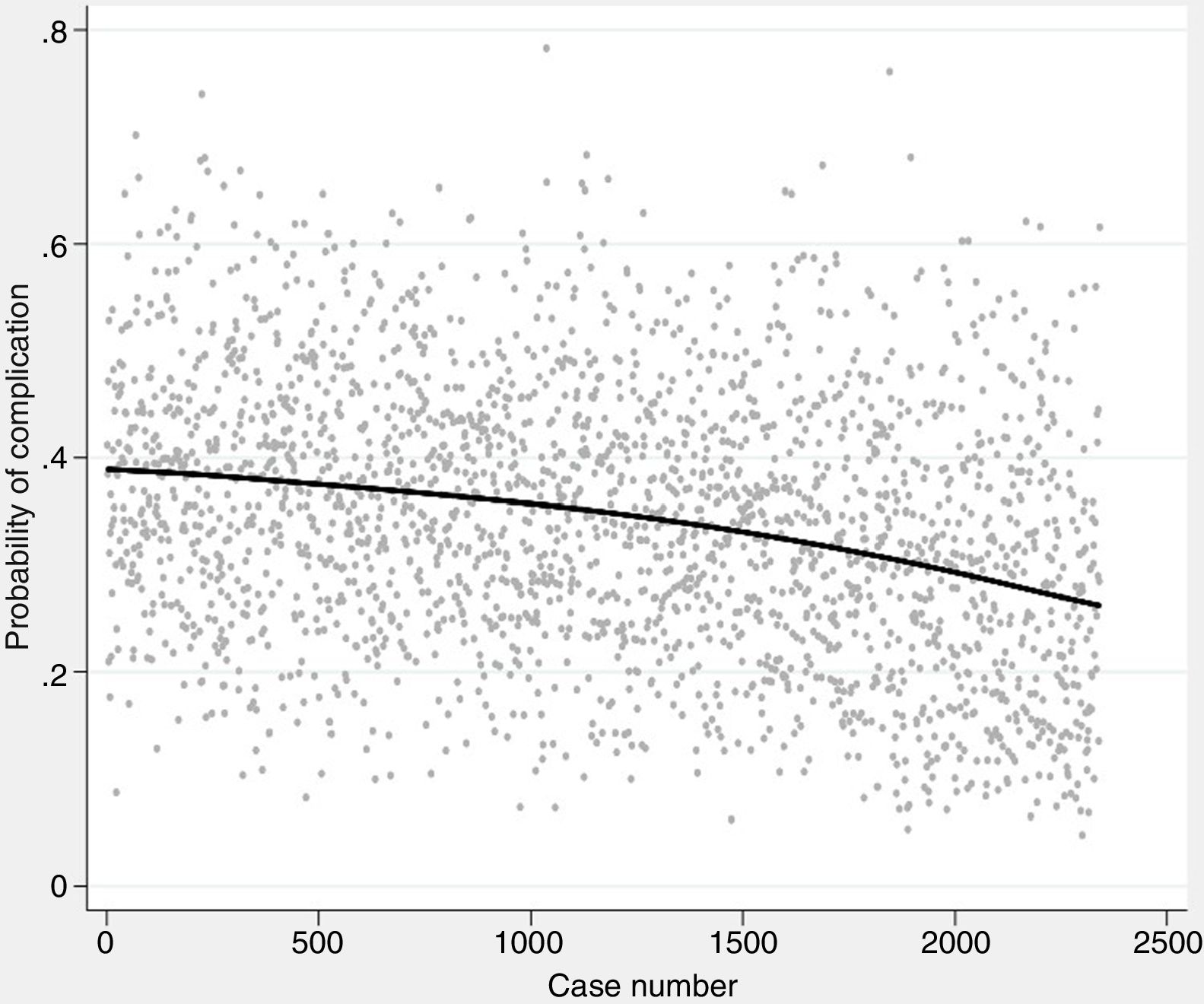
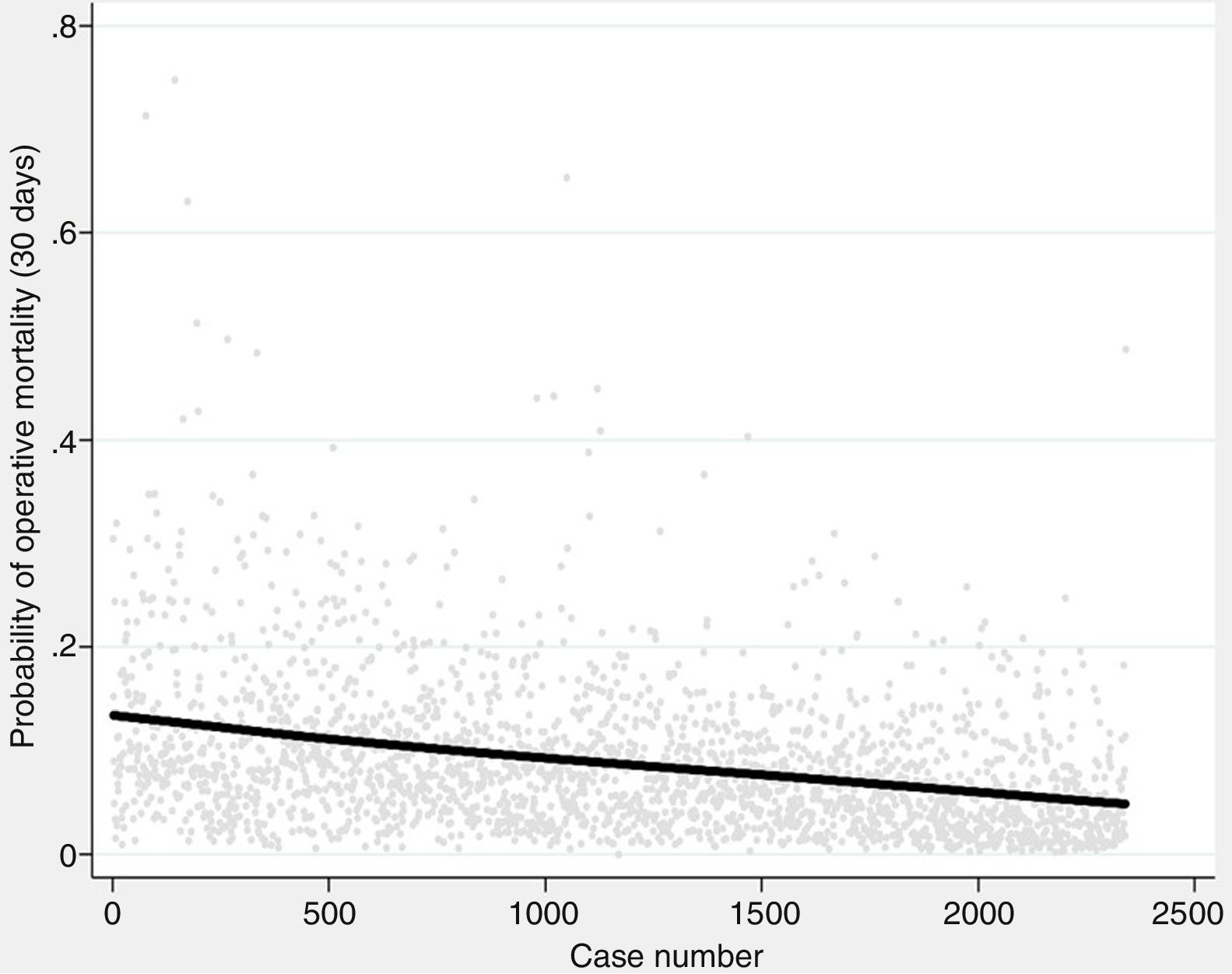
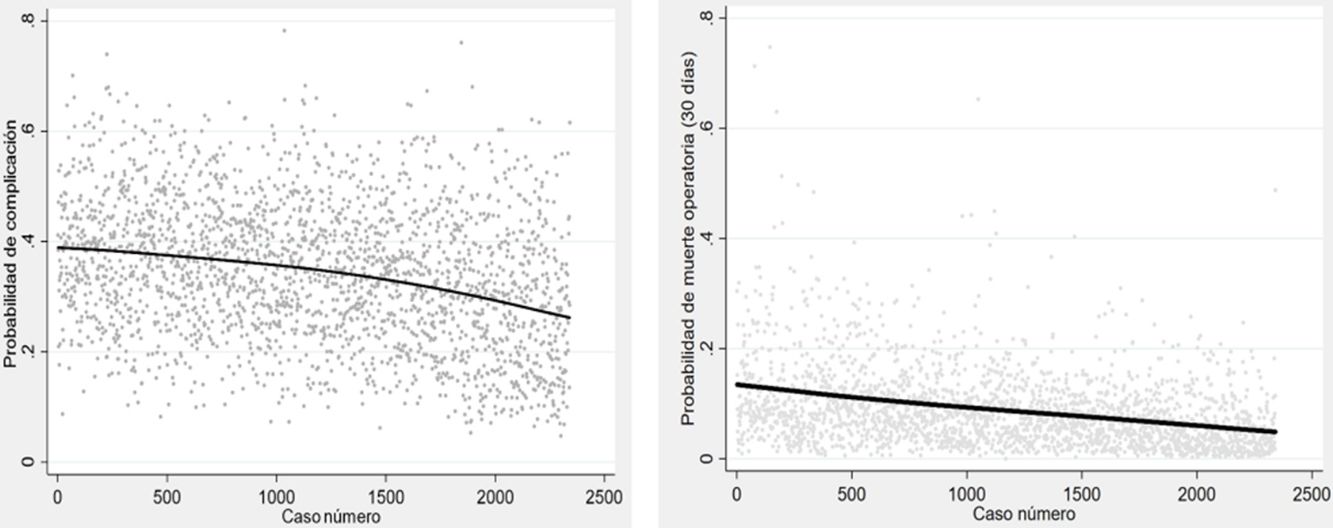
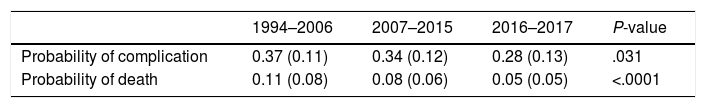
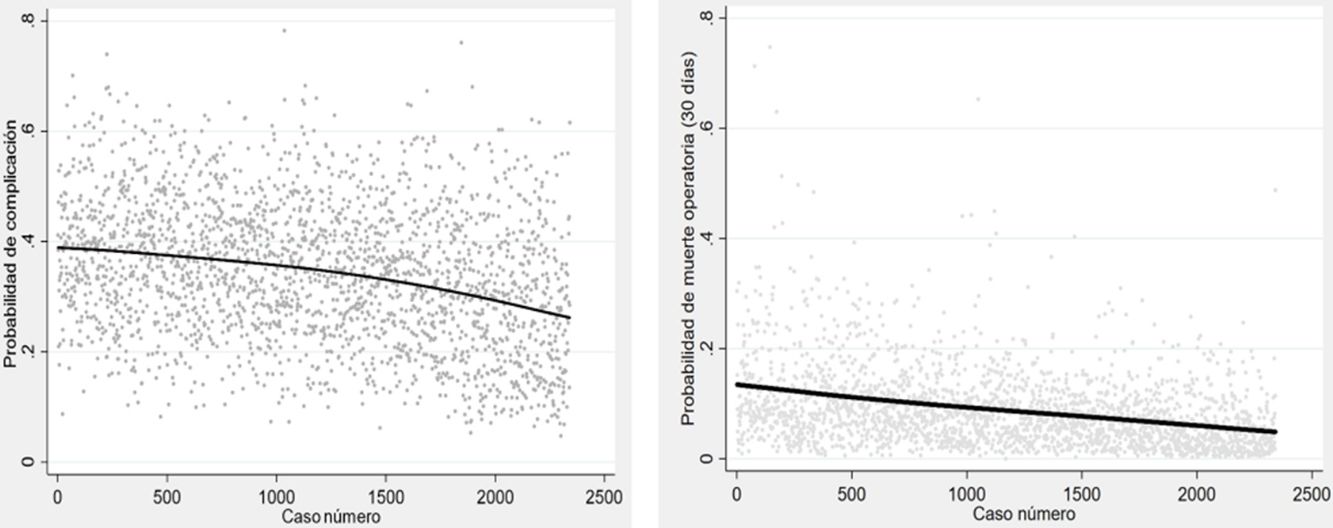
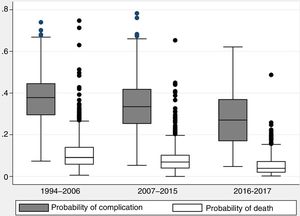
The probability of complications and death after lung resection, calculated using the Eurolung 1 and 2 models, respectively, is shown in Table 1 and in Fig. 1. The probability that the differences between the values are due to chance is <.0001. Figs. 2 and 3 represent the polynomial regression models weighted for morbidity and mortality, respectively, revealing a clear decline in both variables over time.
Probability of Cardiorespiratory Complications and Operative Mortality (at 30 Days) in Each Study Period, Calculated Using the Eurolung 1 and 2 Risk Models, Respectively (Standard Deviation in Parentheses).
| 1994–2006 | 2007–2015 | 2016–2017 | P-value | |
|---|---|---|---|---|
| Probability of complication | 0.37 (0.11) | 0.34 (0.12) | 0.28 (0.13) | .031 |
| Probability of death | 0.11 (0.08) | 0.08 (0.06) | 0.05 (0.05) | <.0001 |
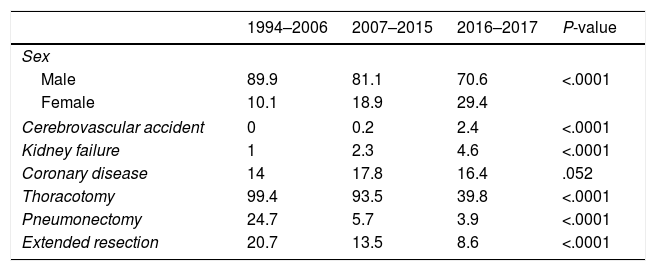
The relative frequency of binary variables in the last period differed from the previous 2 periods, except in the case of CD, which remained unchanged (Table 2). The prevalence of CVA and CKD increased with time, reaching 2.4% and 4.6% in the last period, respectively. With regard to changes in surgical practices, the percentages of pneumonectomy and extended resections in the first and third period fell by more than 20% and 12%, respectively, and the number of cases undergoing VATS increased dramatically in the last period, eventually representing more than 60% of all surgeries performed.
Prevalence (%) of Dichotomous Risk Variables in the 3 Study Periods.
| 1994–2006 | 2007–2015 | 2016–2017 | P-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 89.9 | 81.1 | 70.6 | <.0001 |
| Female | 10.1 | 18.9 | 29.4 | |
| Cerebrovascular accident | 0 | 0.2 | 2.4 | <.0001 |
| Kidney failure | 1 | 2.3 | 4.6 | <.0001 |
| Coronary disease | 14 | 17.8 | 16.4 | .052 |
| Thoracotomy | 99.4 | 93.5 | 39.8 | <.0001 |
| Pneumonectomy | 24.7 | 5.7 | 3.9 | <.0001 |
| Extended resection | 20.7 | 13.5 | 8.6 | <.0001 |
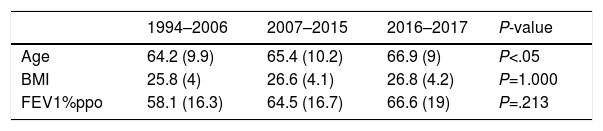
With regard to continuous variables (Table 3), more older patients underwent surgery in the last period compared with previous periods. ppoFEV1% was also greater in the last two periods, compared with the first (Table 3).
DiscussionThe main results of the study show that the probability of 30-day mortality and cardiorespiratory morbidity according to Eurolung 1 and 2 after anatomical lung resection has declined over the past 20 years in our unit. Both risks are lower in the last period compared with the 2 previous periods. This temporal trend is consistent with the mortality and morbidity figures reported by the ESTS in their annual reports.1
The prevalence of variables that influence the risk of death and cardiopulmonary complications has also changed over time. Patients undergoing surgery in the last 2 years were older than in the 2 preceding periods, probably as a result of increased life expectancy of the population. Age is considered by some authors to be an important risk factor for morbidity after lung resection. Patients older than 70 years are more than twice as likely to develop complications after lung resection compared with younger patients.3–5 However, Ogawa et al.6 showed that postoperative complications in elderly patients with lung cancer were independent of age, and Stamenovic et al.7 found that patients older than 80 years did not have a higher risk of developing complications after lung resection than patients in their 70s. Nevertheless, it is important to remember that, in general, there is a selection bias when evaluating octogenarian patients.8 In any case, the average age of patients undergoing lung resection has increased over time without increasing the risk of death and complications, although it should be pointed out that the average age of patients in the third period barely reaches 67 years.
Life expectancy has increased in parallel with the prevalence of comorbidities. Thus, for example, the percentage of patients with a history of CVA and CKD has increased in the last 2 periods compared with the first. Improved management of these diseases has most likely delayed the increase of associated morbidity. CD and BMI, however, have not changed significantly over time. Wang et al.4 found that age (70 years or older) and ppoFEV1% (70% or lower) combined with CD were independent prognostic factors for major postoperative complications. According to our results, ppoFEV1% has increased over time, especially when we compare the last 2 periods with the first. This finding may be explained by the decline in the number of pneumonectomies performed in the last 2 periods.
Surgical practices have also evolved over time: only 4% of the procedures performed in the last period were pneumonectomies. Pneumonectomy continues to be a high-risk procedure that is associated with high rates of mortality and morbidity.9 For this reason, various strategies aimed at preserving the parenchyma have been developed and widely adopted, with the dual objective of ensuring complete resection of the tumor while preserving lung function. As a result, the number of pneumonectomies has fallen dramatically, a trend that is due, among other factors, to an increase in the number of bronchoplastic resections performed and improvements in early detection. These factors were not analyzed in this study.
Fewer extended resections were performed in recent years, probably also due to earlier diagnosis and treatment of lung cancers. Our results show a rate of 8.6% of extended resections in the last 2 years, comparable to the percentage given in the annual ESTS report of 2018.1 A study by Berry et al.5 underlines the importance of extended resections and thoracotomy as relevant risk factors for morbidity after lung resection surgery. The percentage of thoracotomies also decreased significantly in the last period. Several studies10–12 have shown that lobectomy performed via VATS is superior to the open approach in terms of early outcomes, and, more recently, a paired case control study using data from the ESTS registry13 confirmed that lobectomy via VATS is associated with a lower incidence of complications and mortality compared with thoracotomy.
Finally, the percentage of women undergoing anatomical lung resection in the last two years has increased, due in large part to the progressively increasing prevalence of lung cancer in women.14 According to the 2012 report of the Spanish Society of Medical Oncology, the incidence of lung cancer in women was 5.67%.15 The increase in lung cancer incidence and mortality among women reflects changing trends in smoking, which reached its peak among women in the USA almost 20 years later than in men.14 Additionally, after adjusting for the level of tobacco exposure, women have an increased risk of cancer compared with men.14 In contrast, women have lower morbidity and mortality after surgery for lung cancer. The risk of complications is almost 3 times higher in men,3,7,16 because women tend to be younger and have fewer comorbidities than men.17
The increase in the percentage of minimally invasive surgeries and female patients, along with the falling numbers of pneumonectomies and extended resections may be the most important factors that have led to a reduction in the risk of morbidity and mortality after anatomical lung resection.
Study LimitationsThe most important limitation of our research is that the risk models were constructed using data from patients included in the second period. However, our objectives did not include validating the models, so we believe that this limitation does not compromise the study results.
The Eurolung models are the most up-to-date available, but data were not collected on diffusing capacity of the lung for carbon monoxide or maximum oxygen consumption, parameters that are considered predictive of postoperative morbidity and mortality; it might have been interesting to know how these variables have evolved over time and how they have influenced the risks of postoperative morbidity and mortality.
Finally, this study may have the biases inherent to any retrospective analysis. Although complications were well defined and standardized variables were used,18 we cannot rule out data entry errors, misclassification, or insufficient reporting. Indeed, we excluded 4% of our total number of cases due to incomplete data.
ConclusionsThis study provides real data quantifying the decreasing risk of mortality and cardiorespiratory morbidity after anatomical lung resection over time. According to our analysis, this reduction can be attributed to the falling number of pneumonectomies and extended resections (although the cause of this pattern was not analyzed), and the growing use of minimally invasive procedures. Other changes in the clinical characteristics of the patients referred and accepted for surgery do not seem to have influenced the outcomes.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Gómez Hernández MT, Valentín NN, Rodríguez Alvarado I, Fuentes Gago M, Varela Simó G, Jiménez López MF. Modificación del riesgo de mortalidad y morbilidad tras resección pulmonar en los últimos 20 años. Arch Bronconeumol. 2020;56:23–27.