Few studies have comprehensively assessed the evolution of asthma disease in recent years.
ObjectivesTo determine changes in morbidity, lung function and quality of life and to establish the impact in terms of cost in a cohort of patients with asthma.
MethodsProspective, descriptive and realistic study that included 220 asthma patients evaluated 10 years after their inclusion (1994–2004). For all the patients, data for symptoms, lung function, quality of life and financial cost were collected.
ResultsThere was a decrease in the frequency of health service visits, including: emergency room visits for asthma exacerbations, 0.3 (0.9) versus 0.6 (1) visits per patient per year (P=.003); a reduction in the severity of the disease, with a greater proportion of patients with mild asthma, 121 (54.8%) versus 94 (42.7%) (P=.001); a decrease (improvement in quality of life) in the total SGRQ, 30.1 (16.5) versus 37 (19.6) (P<.001); and reduced total costs, 1464€ (3415.8) compared to 2267€ (4174) per patient/year (P<.001), mainly due to indirect costs, 617.50€ (2855.9) compared to 1320.10€ (3685.3) per patient/year (P=.001). When assessing the changes observed according to asthma severity, no differences were observed between groups.
ConclusionsThe evolution of the morbidity and quality of life of asthma patients between 1994 and 2004 are clearly favorable. This improvement provided a significant reduction in the total costs of disease treatment.
Son escasos los estudios que han evaluado de forma global la evolución de la enfermedad asmática en los últimos años.
ObjetivosDeterminar los cambios en la morbilidad, la función pulmonar y la calidad de vida, y establecer el impacto, en términos económicos, de una cohorte de pacientes con asma.
MétodoEstudio prospectivo, realístico y descriptivo que incluyó 220 asmáticos evaluados a los 10 años de su inclusión (1994–2004). Se recogieron datos clínicos, de función pulmonar, de calidad de vida y de costes económicos.
ResultadosSe observó un descenso en la frecuentación de los servicios sanitarios, entre otros el de las visitas en urgencias por exacerbación asmática, 0,3 (0,9) por 0,6 (1) visitas por paciente/año (p=0,003); una reducción de la gravedad de la enfermedad, con una mayor proporción de pacientes con asma leve, 121 (54,8%) frente a 94 (42,7%) (p=0,001); un descenso (mejoría de la calidad de vida) en la puntuación total del cuestionario de St. Georges, 30,1 (16,5) frente a 37 (19,6) (p<0,001), y una reducción de los costes totales, 1.464€ (3.415,8) por 2.267€ (4.174) paciente/año (p<0,001), fundamentalmente a expensas de los costes indirectos, 617,5€ (2.855,9) frente a 1.320,1€ (3.685,3) paciente/año (p=0,001). Al considerar los cambios observados en función del nivel de gravedad, no se constataron diferencias entre los grupos, mejorando todos por igual.
ConclusionesLa evolución de la morbilidad y de la calidad de vida de los pacientes con asma entre 1994 a 2004 años es notoriamente favorable. Dicha mejora se traduce en una importante reducción de los costes económicos ocasionados por la enfermedad.
According to the World Health Organization, asthma is the seventh most prevalent disease in the world, affecting more than 300 million people. It is a chronic respiratory disease that affects all age groups, from newborns to the elderly. In Spain, although there is certain variability depending on the geographical area considered, it is estimated that around 4% of the adult population is affected.1,2 In addition, due to causes that have not been well established, said prevalence has increased considerably in recent years, particularly in economically developed countries.3 All these logically result in a great consumption of health-care resources. In some countries, the diagnostic and therapeutic management of the disease represents between 1% and 2% of total health-care service expenses.4
These pessimistic numbers are countered by others that are more positive. Recent data on the disease confirm a notable reduction in mortality as well as in frequency of hospital care due to asthma.5,6 It is considered that the causes of said reduction could be related with the possible improvement in the attention given by health-care professionals,5 the extensive diffusion and the impact of the guidelines for clinical practice in asthma,7,8 and particularly by the greater use of inhaled corticosteroids.9 In addition to the favorable effects of this group of drugs, we must also take into account the appearance of the new formulations of the last 15–20 years: long-acting β2-adrenérgic agonists combined in one single inhaler with corticosteroids,10 and also leukotriene receptor antagonists.
Nevertheless, there is limited information available on the recent natural history of asthma in standard clinical practice. Specifically, in our setting there are no longitudinal studies in significant patient samples analyzing the predictable changes in morbidity and quality of life over the last 20 years. Along the same lines, there are no studies that have evaluated the impact that the possible changes in morbidity and new treatments could have on the total costs of the disease.
In said context, the cohort of patients known as “asthma in Osona” represents the ideal framework for responding to the questions posed. This group of patients, who have been followed up without interruption by the same group of professionals for twenty years, have provided valuable information in the past about different clinical and economic aspects related with the disease.11,12 From this standpoint, the objective of the present study was to determine, in the mentioned patient cohort, the magnitude of the evolutionary changes in their disease in terms of morbidity and mortality, lung function, quality of life and costs during the ten-year period from 1994 to 2004.
Materials and MethodsA prospective, longitudinal, descriptive, realistic study designed in order to determine changes in morbidity and mortality, lung function, quality of life and the costs of a cohort of patients with asthma observed for 10 years (1994–2004). It was carried out in the district of Osona, a semi-rural area in the province of Barcelona (Spain), with some 150,000 inhabitants. The study protocol was approved by the Clinical Research Ethics Committee of the Hospital General de Vic, and all the patients granted their consent to participate.
The study entails a follow-up of a patient cohort with asthma whose methods have been previously published.11,12 Briefly, the patients included were over the age of 14 and diagnosed with asthma according to the ATS criteria.13 Their disease intensity was classified according to initial severity (1992 NIH Consensus).14 The patients were seen routinely at the primary-care level and once a year at the outpatient consultations of the pneumology department of the hospital, where the degree of control was determined and the treatment adjusted.
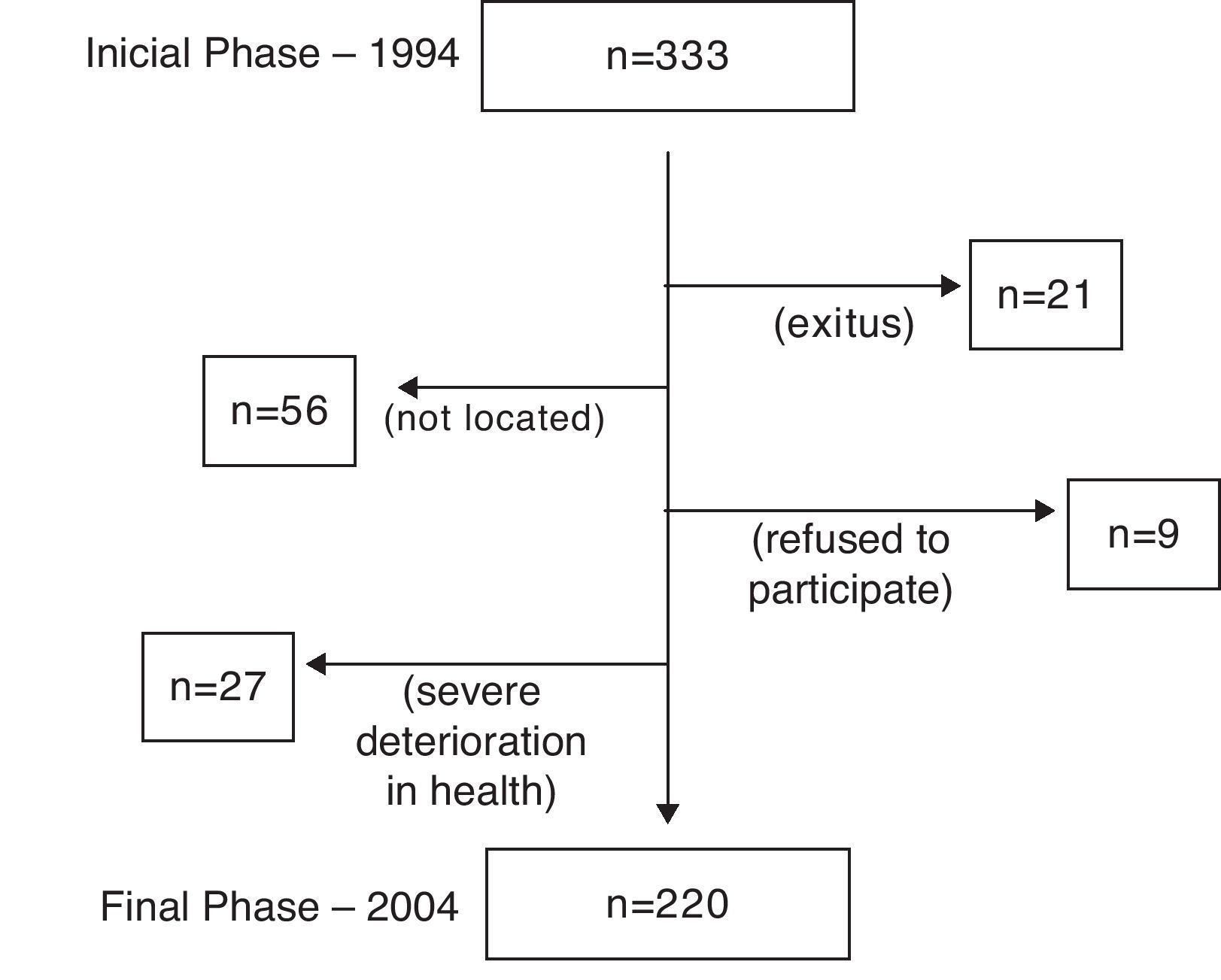
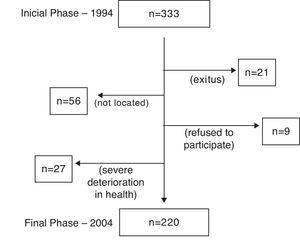
Out of the 330 patients initially evaluated in phase I (1994), 220 (66.7%) were evaluable in phase II (2004). The causes of the 110 losses are compiled in Fig. 1. For the patients who had died over the follow-up period, the cause of death was determined by means of family interview and review of the patient medical files and reports.
Out of a total of 220 patients who were evaluable in the end, both in phase I (1994) as well as in phase II (2004), the following data were compiled and grouped into four categories:
- •
Clinical symptoms and morbidity caused by asthma during the previous year: number of visits to the primary care physician, visits to the emergency room, hospitalizations, days of absenteeism from work, short cycles of oral steroids and standard maintenance treatment received. The information was obtained both from the patient as well as from the medical files.
- •
Spirometric flow by means of spirometry (Datospir-92, Sibelmed, Barcelona, Spain) before and after the administration of inhaled salbutamol.15
- •
Quality of life, determined with the supervised completion of the Spanish version of the St. George's Respiratory Questionnaire (SGRQ).16,17
- •
Economic costs, using a specially designed questionnaire that compiled the direct costs (medication, office visits, emergency room visits, hospitalizations and complementary tests) and indirect costs (missed work, invalidity) caused by the disease in the year prior to the data collection.
The economic costs ten years later were updated in accordance with the Finance Department of the Hospital General de Vic, and the days of work missed and disability were based on the data from the National Institute of Statistics (Instituto Nacional de Estadística – INE).18,19 In both phases of the study, the same researcher (AC) was in charge of interviewing the patients, performing the spirometries and administering the SGRQ.
Statistical AnalysisA descriptive analysis was completed for the variables collected from both phases of the study. The values were expressed as means and standard deviation (SD) or, if necessary, as number of cases with their percentage. The results of the three asthma severity groups were compared using the (2 or Fisher's exact test for the qualitative variables, or rather with the Kruskal–Wallis test for the quantitative variables, depending on their distribution. The changes observed between the two phases were expressed as a difference between the means and were analyzed with the Wilcoxon test for the quantitative variables; for the qualitative variables, the McNemar test was used. The Kolmogorov–Smirnov test was used to check whether the distribution of a variable could be considered normal or not. The differences with a P-value <.05 were considered statistically significant. The information compiled was input after double-checking and was analyzed in a database with SPSS version 12 software (SPSS-PC, Chicago, IL, USA).
ResultsDuring the 10-year follow-up of the cohort, 21 (6.3%) of the patients evaluated at the beginning had died. Only one case of death was caused by a fatal asthma episode. The causes of the remaining deaths were: eight cases of neoplasm (three gastrointestinal, two pulmonary, one urinary, one bone and one of undetermined neoplastic etiology); five due to cardiovascular diseases (two cerebrovascular accidents, two refractory heart failures and one myocardial infarction); four due to severe respiratory infections; one due to evolved hepatic cirrhosis; another due to biliary sepsis, and finally one more due to trauma (traffic accident).
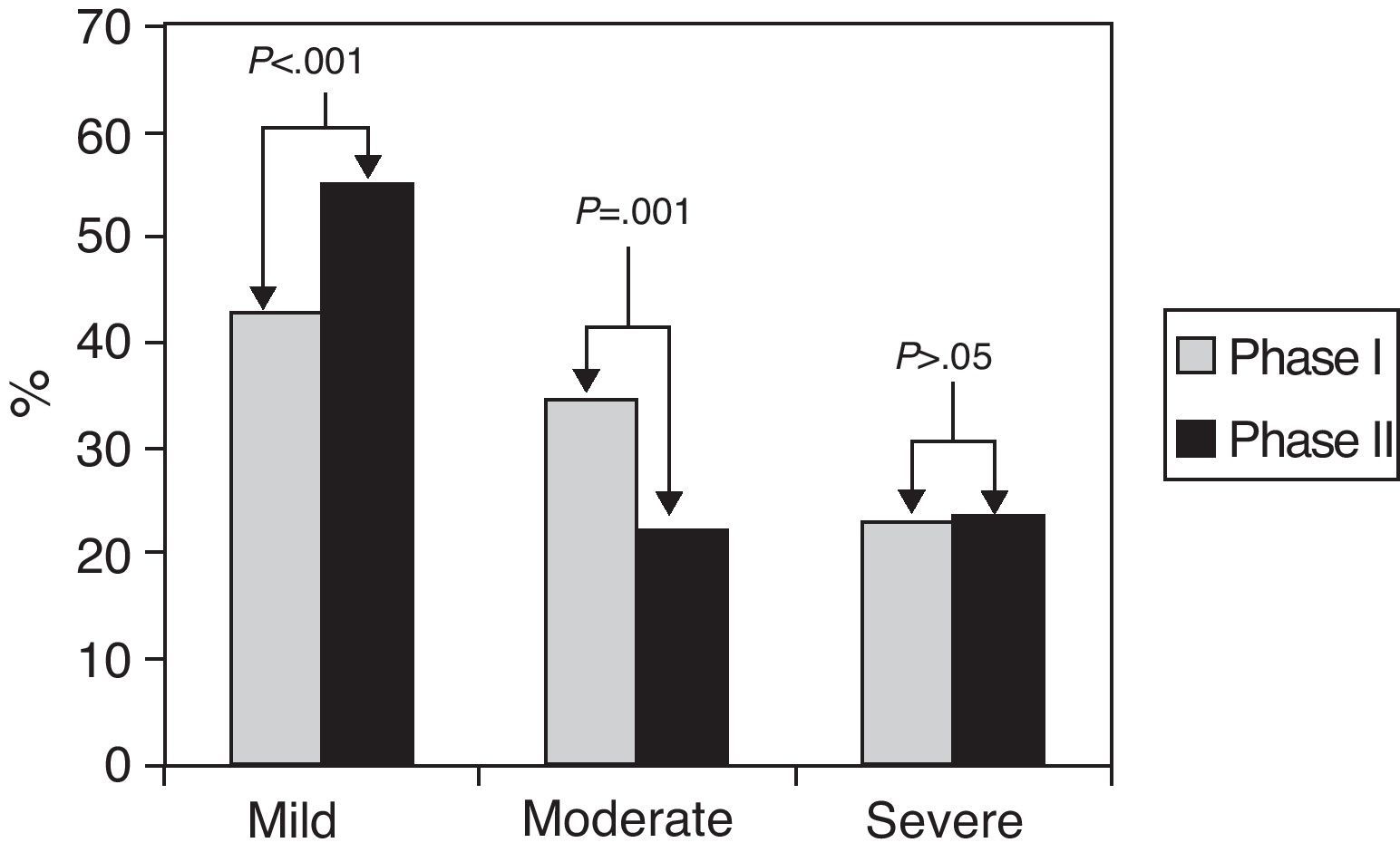
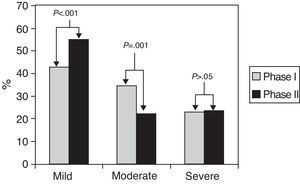
Fig. 2 shows the distribution of asthma severity in both phases. When we compared the proportion of the different levels of severity between both determinations, we observed that in 151 (69%) patients there were no changes, 46 (21%) improved and 23 (10%) worsened. However, when the said changes were considered as a whole, there was a confirmed improvement in general asthma severity 10 years later, with a significantly greater proportion of patients with mild asthma and a reduction of moderate asthma in phase II compared with asthma in phase I.
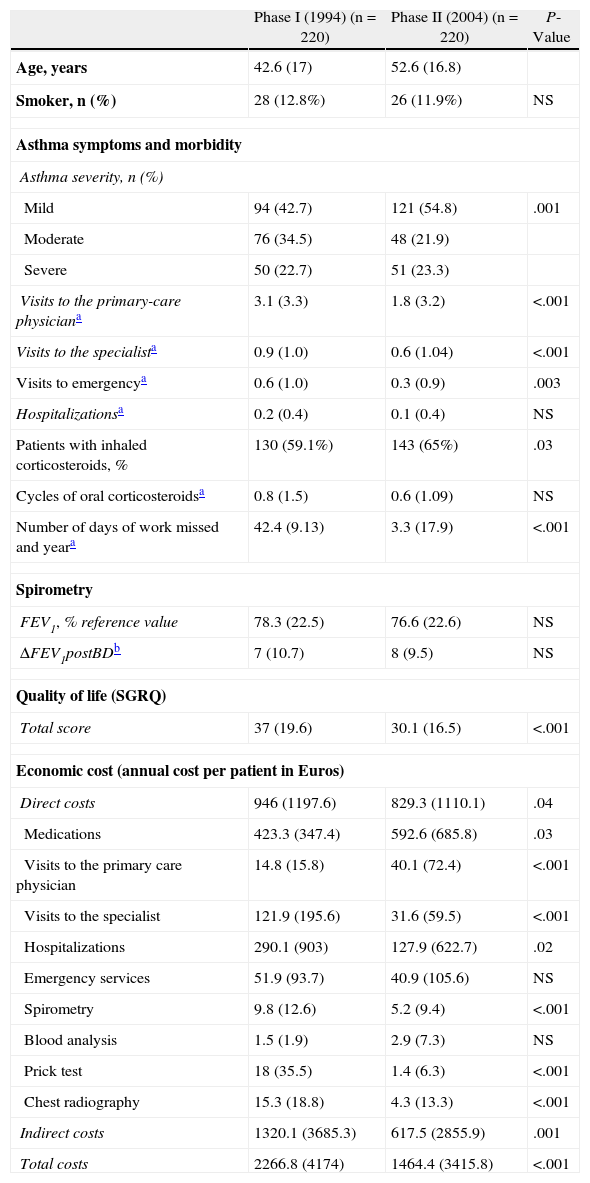
Table 1 compiles the results of the 220 (142 [64.5%] women) patients followed during the 10 years. The comparison between both phases revealed a general improvement in phase II (2004) compared with phase I (1994). Thus, among the clinical and morbidity variables for asthma during the previous year, there was a significant reduction in the number of office visits in primary care, specialized care and the emergency department as well as the number of missed work days. At the same time, the use of inhaled corticosteroids increased significantly and the use of their combination with long-acting (2-adrenergic agonists was introduced: in 2004, 21% of the patients were receiving them, while in 1994 none received, as the drug was not being commercialized still (data not shown in Table 1). The total SGRQ score was significantly lower and, therefore, quality of life improved. In contrast with the earlier results, there was an observed non-significant decline in the mean FEV1. The economic expense analysis showed statistically significant changes in the reduction of the total costs, both direct and indirect. Except for the expenses incurred due to the purchase of medication and primary-care office visits, both of which increased, the rest of the different categories that make up the direct costs (except those caused by blood analyses, which remain unchanged) were significantly reduced.
Clinical Treatment and Morbidity of Asthma, Lung Function, Quality of Life and Costs Compiled in Phase I (1994) and in Phase II (2004) of the Study.
| Phase I (1994) (n=220) | Phase II (2004) (n=220) | P-Value | |
| Age, years | 42.6 (17) | 52.6 (16.8) | |
| Smoker, n (%) | 28 (12.8%) | 26 (11.9%) | NS |
| Asthma symptoms and morbidity | |||
| Asthma severity, n (%) | |||
| Mild | 94 (42.7) | 121 (54.8) | .001 |
| Moderate | 76 (34.5) | 48 (21.9) | |
| Severe | 50 (22.7) | 51 (23.3) | |
| Visits to the primary-care physiciana | 3.1 (3.3) | 1.8 (3.2) | <.001 |
| Visits to the specialista | 0.9 (1.0) | 0.6 (1.04) | <.001 |
| Visits to emergencya | 0.6 (1.0) | 0.3 (0.9) | .003 |
| Hospitalizationsa | 0.2 (0.4) | 0.1 (0.4) | NS |
| Patients with inhaled corticosteroids, % | 130 (59.1%) | 143 (65%) | .03 |
| Cycles of oral corticosteroidsa | 0.8 (1.5) | 0.6 (1.09) | NS |
| Number of days of work missed and yeara | 42.4 (9.13) | 3.3 (17.9) | <.001 |
| Spirometry | |||
| FEV1, % reference value | 78.3 (22.5) | 76.6 (22.6) | NS |
| ΔFEV1postBDb | 7 (10.7) | 8 (9.5) | NS |
| Quality of life (SGRQ) | |||
| Total score | 37 (19.6) | 30.1 (16.5) | <.001 |
| Economic cost (annual cost per patient in Euros) | |||
| Direct costs | 946 (1197.6) | 829.3 (1110.1) | .04 |
| Medications | 423.3 (347.4) | 592.6 (685.8) | .03 |
| Visits to the primary care physician | 14.8 (15.8) | 40.1 (72.4) | <.001 |
| Visits to the specialist | 121.9 (195.6) | 31.6 (59.5) | <.001 |
| Hospitalizations | 290.1 (903) | 127.9 (622.7) | .02 |
| Emergency services | 51.9 (93.7) | 40.9 (105.6) | NS |
| Spirometry | 9.8 (12.6) | 5.2 (9.4) | <.001 |
| Blood analysis | 1.5 (1.9) | 2.9 (7.3) | NS |
| Prick test | 18 (35.5) | 1.4 (6.3) | <.001 |
| Chest radiography | 15.3 (18.8) | 4.3 (13.3) | <.001 |
| Indirect costs | 1320.1 (3685.3) | 617.5 (2855.9) | .001 |
| Total costs | 2266.8 (4174) | 1464.4 (3415.8) | <.001 |
Values expressed in means (standard deviation), except when indicated in the table as number of cases (percentage).
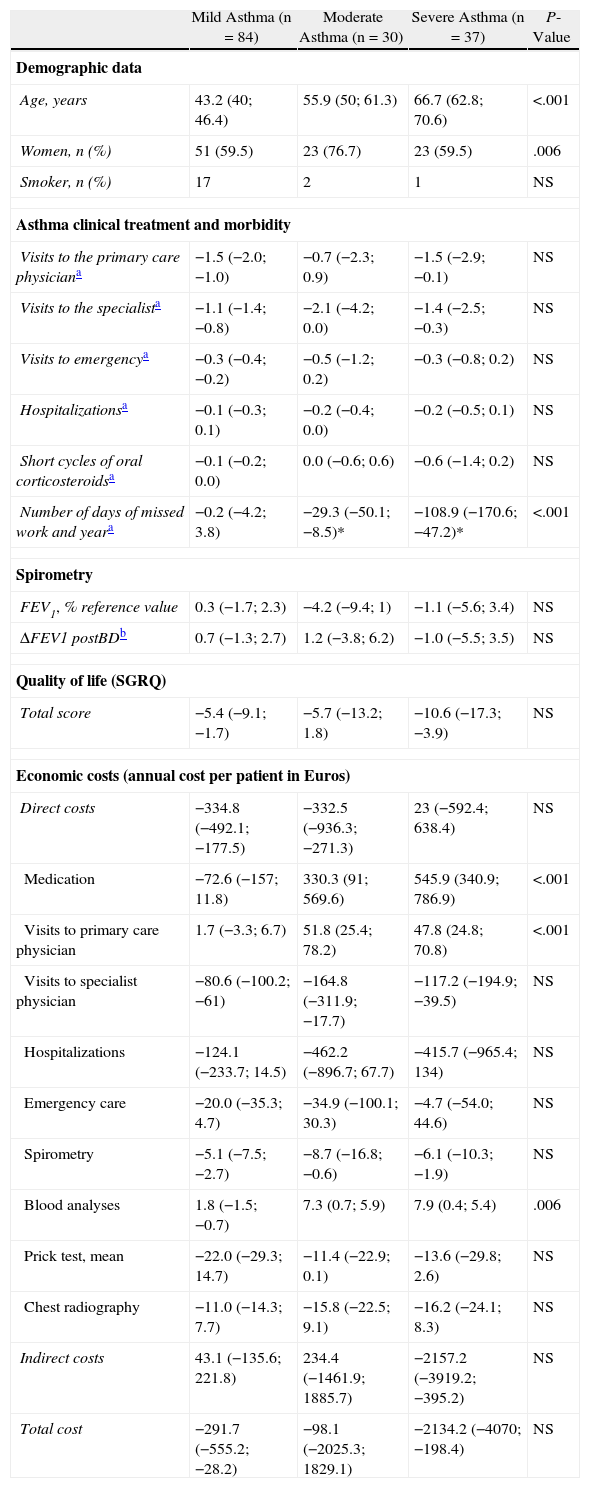
With the aim of evaluating the possible different magnitude in the changes observed between the two phases of the study according to the level of asthma severity, the sample analyzed was subdivided into the three levels of severity proposed in the International Asthma Consensus of the NIH in 1992: mild, moderate and severe asthma.14 In order to avoid possible confusion in the grouping of the cases, we excluded from the following analysis those patients who had changed in level of severity in phase II. Therefore, in the end we evaluated with the defined criteria only those data obtained from the 151 patients who did not change in asthma severity over the course of the entire study. Table 2 shows in each severity group the difference of the means between phase I (1994) and phase II (2004) of the variables analyzed in the 151 patients mentioned. The analysis verifies the improvement of the results of the variables studied in each one of the three levels of severity, with negative values as the general morbidity and costs declined between the two phases. And, although in some variables (primary care physician visits, short cycles of corticosteroids, work absenteeism, SGRQ and indirect costs) there is a tendency towards a greater reduction (or improvement) in severe asthma, when compared with mild or moderate asthma, only days of work absenteeism reached statistical significance.
Comparison of the Magnitude of the Changes Observed at Each Level of Asthma Severity in the two Phases of the Study. Differences in the Averages Between Phase I (1994) and Phase II (2004) of the Clinical and Morbidity Variables of Asthma, Lung Function, Quality of Life and Costs for the 151 Patients that did not Vary in Severity Level Between Phases.
| Mild Asthma (n=84) | Moderate Asthma (n=30) | Severe Asthma (n=37) | P-Value | |
| Demographic data | ||||
| Age, years | 43.2 (40; 46.4) | 55.9 (50; 61.3) | 66.7 (62.8; 70.6) | <.001 |
| Women, n (%) | 51 (59.5) | 23 (76.7) | 23 (59.5) | .006 |
| Smoker, n (%) | 17 | 2 | 1 | NS |
| Asthma clinical treatment and morbidity | ||||
| Visits to the primary care physiciana | −1.5 (−2.0; −1.0) | −0.7 (−2.3; 0.9) | −1.5 (−2.9; −0.1) | NS |
| Visits to the specialista | −1.1 (−1.4; −0.8) | −2.1 (−4.2; 0.0) | −1.4 (−2.5; −0.3) | NS |
| Visits to emergencya | −0.3 (−0.4; −0.2) | −0.5 (−1.2; 0.2) | −0.3 (−0.8; 0.2) | NS |
| Hospitalizationsa | −0.1 (−0.3; 0.1) | −0.2 (−0.4; 0.0) | −0.2 (−0.5; 0.1) | NS |
| Short cycles of oral corticosteroidsa | −0.1 (−0.2; 0.0) | 0.0 (−0.6; 0.6) | −0.6 (−1.4; 0.2) | NS |
| Number of days of missed work and yeara | −0.2 (−4.2; 3.8) | −29.3 (−50.1; −8.5)* | −108.9 (−170.6; −47.2)* | <.001 |
| Spirometry | ||||
| FEV1, % reference value | 0.3 (−1.7; 2.3) | −4.2 (−9.4; 1) | −1.1 (−5.6; 3.4) | NS |
| ΔFEV1 postBDb | 0.7 (−1.3; 2.7) | 1.2 (−3.8; 6.2) | −1.0 (−5.5; 3.5) | NS |
| Quality of life (SGRQ) | ||||
| Total score | −5.4 (−9.1; −1.7) | −5.7 (−13.2; 1.8) | −10.6 (−17.3; −3.9) | NS |
| Economic costs (annual cost per patient in Euros) | ||||
| Direct costs | −334.8 (−492.1; −177.5) | −332.5 (−936.3; −271.3) | 23 (−592.4; 638.4) | NS |
| Medication | −72.6 (−157; 11.8) | 330.3 (91; 569.6) | 545.9 (340.9; 786.9) | <.001 |
| Visits to primary care physician | 1.7 (−3.3; 6.7) | 51.8 (25.4; 78.2) | 47.8 (24.8; 70.8) | <.001 |
| Visits to specialist physician | −80.6 (−100.2; −61) | −164.8 (−311.9; −17.7) | −117.2 (−194.9; −39.5) | NS |
| Hospitalizations | −124.1 (−233.7; 14.5) | −462.2 (−896.7; 67.7) | −415.7 (−965.4; 134) | NS |
| Emergency care | −20.0 (−35.3; 4.7) | −34.9 (−100.1; 30.3) | −4.7 (−54.0; 44.6) | NS |
| Spirometry | −5.1 (−7.5; −2.7) | −8.7 (−16.8; −0.6) | −6.1 (−10.3; −1.9) | NS |
| Blood analyses | 1.8 (−1.5; −0.7) | 7.3 (0.7; 5.9) | 7.9 (0.4; 5.4) | .006 |
| Prick test, mean | −22.0 (−29.3; 14.7) | −11.4 (−22.9; 0.1) | −13.6 (−29.8; 2.6) | NS |
| Chest radiography | −11.0 (−14.3; 7.7) | −15.8 (−22.5; 9.1) | −16.2 (−24.1; 8.3) | NS |
| Indirect costs | 43.1 (−135.6; 221.8) | 234.4 (−1461.9; 1885.7) | −2157.2 (−3919.2; −395.2) | NS |
| Total cost | −291.7 (−555.2; −28.2) | −98.1 (−2025.3; 1829.1) | −2134.2 (−4070; −198.4) | NS |
Values expressed as differences from the means for each level of severity (95% confidence interval), except when indicated in the table as number of cases (percentage).
The main contribution of the present study is the confirmation that the clinical evolution of asthmatic disease in recent years is notoriously favorable, with a significant reduction in morbidity, an improvement in the quality of life of the patients and a substantial reduction in total. Also, these changes are independent from the initial level of severity, even including the severest forms of the disease. The improvement coincides with the increased use of inhaled corticosteroids, the introduction of the combinations of corticosteroids and long-acting (2-adrenergic agonists, and presumably (as they coincide in time) with the publishing of clinical practice guidelines.7,14 Furthermore, it is important to highlight the dimension of the changes observed, which provided a considerable reduction (50%) in the rate of emergency department visits and hospitalizations and a decline of 7 points in the SGRQ, with a tendency towards being more notable in the moderate and severe disease types. Consequently, there was a spectacular reduction in the number of days of work absenteeism associated with asthma.
In general, the results concur with those observed in other international studies with similar designs.5,20 Among these are the so-called “Finnish experience” (The Finnish Asthma Programme), in which, after the implementation of an ambitious nation-wide program, they confirmed 10 years after its application (1993–2003) a significant and considerable reduction in morbidity and mortality (particularly in severe exacerbations, hospitalizations and death) and total costs.5
Recent national21,22 and international23 studies have consistently demonstrated that only between 33% and 55% of the patients with asthma are appropriately controlled. A priori, the satisfactory results of our study could go against the widespread opinion of the current insufficient control of asthma. The explanation of the supposed incongruence between both affirmations lies in the fact that, although therapeutic improvements have provided a substantial reduction in morbidity and mortality and an improvement in the quality of life of the patients, they have not been able to promote a less-demanding morbidity, such as that of well-controlled asthma. This circumstance could possibly be related with the limited use of educational programs. A survey carried out in Spain that interviewed more than 1000 physicians and nurses, who are usually involved in the follow-up of asthma patients, revealed that only 16% of those interviewed declared that a standardized, structured education program was used in their health-care centers.24
Observations made in large patient samples or by using meta-analyses of clinical trials associated the use of long-acting (2-adrenergic agonists with an infrequent but significantly greater risk of death and severe exacerbations.25,26 With said premise, our study should have identified an increase (or at least not show changes) in exacerbations and hospitalizations, as one-fifth of the sample analyzed (21%) were taking them in phase II (2004), compared with phase I (1994) when no patients were. Contrarily, a significant reduction was observed in said parameters, even in the severest patients. These results agree with the growing opinion contrary to the supposed deleterious effect of long-acting (2-adrenergic agonists27 and are in tune with another study recently done in our setting.10
Among the results of the study, we found striking the non-significant decline in mean FEV1 (−1.3%) observed when comparing the two phases. This reduction contrasts with the favorable results observed in the rest of the clinical variables analyzed. Nevertheless, it is well-known that the asthmatic population experiences an accelerated progressive loss in lung capacity compared with the non-asthmatic population.10 This deterioration is only partially prevented by corticosteroid treatment, which evidently differs with the beneficial action that said drugs have on clinical variables or indicators.28 On the other hand, a greater loss in lung function has been associated with patients who suffer frequent asthma exacerbations, a circumstance attributed to the phenomenon of bronchial remodeling that accompanies the exacerbation.29 Along this line, we should indicate that our study, in agreement with others,20 verified a non-significant tendency in the mild asthma group towards preserving FEV1 (0.3%) compared with the decline observed in those with moderate and severe asthma (−4.2 and −1.1%, respectively). Moreover, these are groups that presented a greater tendency towards exacerbations (visits to emergency and hospitalization) due to asthma.
The analysis of the economic data of the study revealed that, in agreement with the lower morbidity and particularly the decline in hospital care, the total costs decreased significantly. The mean of the total cost per patient registered in 2004 represented a decrease of 35% over the average of 1994. These data are equivalent to that observed in the Finnish experience, where the application of their national program provided a reduction of 36% in total costs.5 The mean total cost in our study was 1464.40€, which also does not differ substantially from another study recently done in a Spanish sample with 627 patients (ASMACOST study),30 which established this amount at 1533€. The reduction in total costs observed in the present study came from both the direct as well as the indirect costs. Regarding the direct costs, although there was a statistically significant increase in the expense caused by the purchase of drugs and primary care office visits in 2004 compared to 1994, the decrease in other direct costs—particularly those related with hospitalizations, emergency department visits and specialized care—resulted in a significant reduction in the sum of the direct costs. Along the same lines, the decline in the number of days of work missed provided a significant and considerable reduction, somewhat more than half, of the indirect costs. These results are particularly relevant as some pharmacoeconomic studies usually present partial cost analyses, sometimes elaborated by the health-care administrations themselves, excluding from the evaluation the indirect costs and those related with the frequency of health-care required. It is an improper procedure because in this manner the impact provided by the efficiency of the medication for better controlling a disease, as happens in asthma, cannot be evaluated in the dimension that cost analyses require. Other studies show results that are equivalent to those of this present study, also finding an increase in the costs for medication, but a reduction in direct and total costs.5
As for the potential limitations of the study, the quality of the results could be questioned as they are obtained from a cohort with 33.3% of lost cases. Nevertheless, said loss is within reason as it is a study carried out over a prolonged period of time. In addition, this percentage is even less than those of other series with similar designs.20,31 Therefore, in our opinion, the loss of cases in this study does not limit its validity or the extent of its conclusions.
In short, the present study covers a lack of local information about the natural history of asthma in actual clinical practice situations. The results demonstrate a favorable evolution of the patients with asthma in our setting in recent years. This improvement is supported by a considerable reduction of the frequency of health-care resources used, an important increase in the quality of life of the affected patients and, consequently, a notable reduction in total costs caused by asthma. Even though this observation can probably be extrapolated to the rest of the Spanish asthmatic population, it would be recommendable to analyze data from studies with similar designs and objectives from different geographical locations of our country.
We would like to thank Dr. Carlos Badiola and Dr. Alejandro Pedromingo for their technical help.
This study was funded in part by GSK (Spain).
Please cite this article as: Serra Batlles J, et al. Cambios en la clínica, la función pulmonar, la calidad de vida y los costes en una cohorte de pacientes asmáticos seguidos durante 10 años. Arch Bronconeumol. 2011;47:482–7.