Guidelines are intended to provide a framework for patient management based on the best available evidence.1 Most, but not all guidelines, incorporate a form of objective evidence gathering, usually a systematic review and a synthesis and evaluation of relevant evidence leading to clinical recommendations.2 The methodology of guidelines has evolved over the past 10–15 years from guidelines that are primarily based on expert opinion (“eminence based medicine”) to recommendations that are now rigorously justified using objective methodology.1 Each approach has its own strengths and weaknesses. The GRADE methodology that has been adopted by the majority of international societies such as the European Respiratory Society (ERS), American Thoracic Society and regulatory bodies such as the National Institute for Health and Care Excellence (NICE) in the United Kingdom incorporates clinical questions based on the PICO (patient, intervention, comparator, outcome) method, systematic review of evidence, meta-analysis for key outcomes and formalised evidence to decision frameworks.1–3 Strengths of this approach is that it is more resistant to intrinsic “expert biases” and limits the ability to make recommendations in the absence of evidence. This has the downside, however, that in some cases physicians may be left without any guidance on a key area of management because insufficient evidence was available.4 The rigour of the GRADE methodology means that only a small number of questions can be addressed in most guidelines and so such guidelines are not usually fully comprehensive.5
Writing guidelines for bronchiectasis are particularly challenging because of a lack of large randomised controlled trials and a sparce literature for addressing key clinical questions.6–8 The quality of management of bronchiectasis, when measured against objective criteria is very poor. Audits in the UK, Italy and elsewhere suggest the majority of patients do not receive basic components of care such as sputum cultures, access to airway clearance techniques or testing for the underlying cause of bronchiectasis.9,10 There is therefore a desperate need for clear recommendations to guide patient management (Table 1).
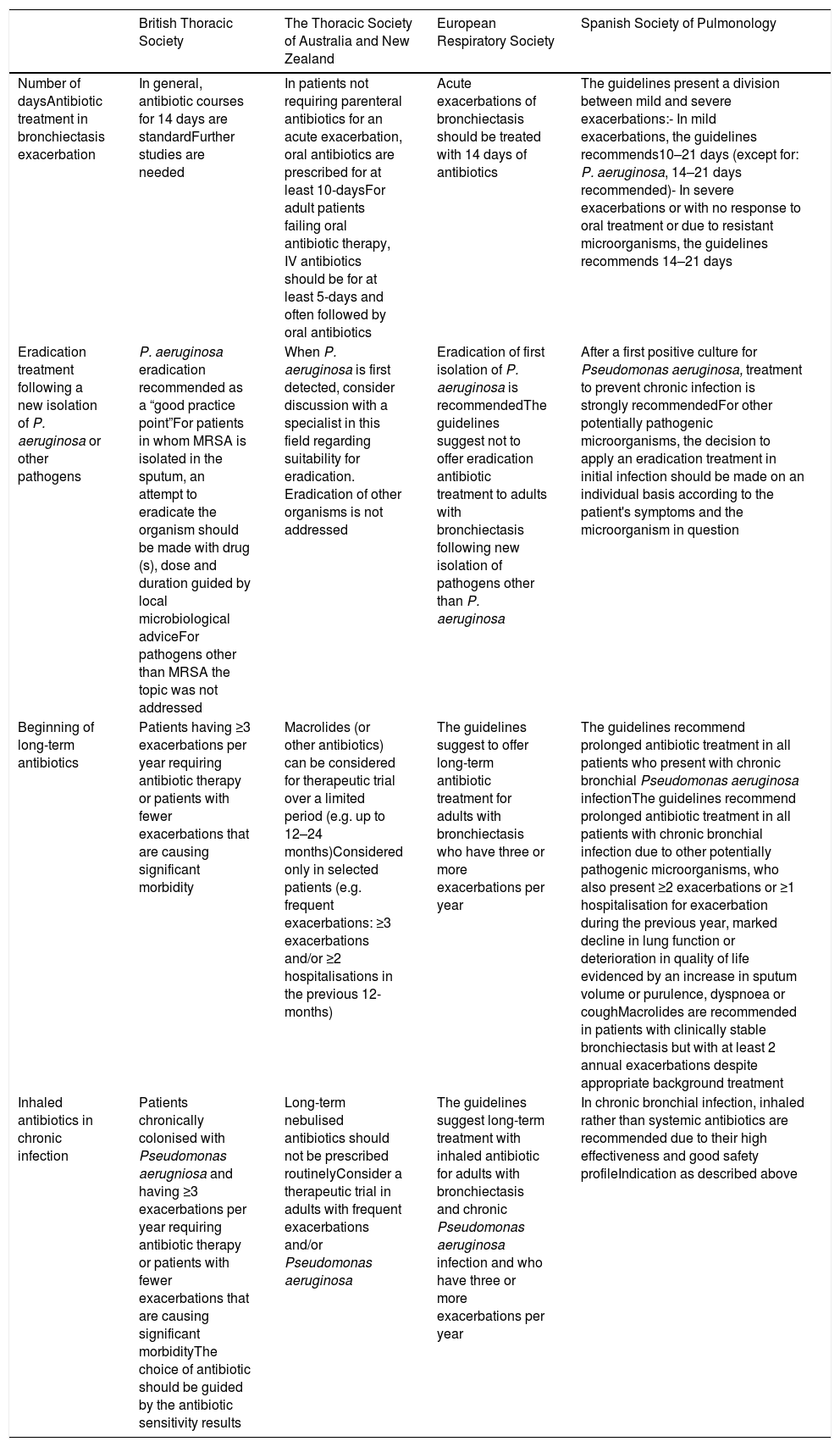
Comparison of Guideline Recommendations in Different Bronchiectasis Guidelines.
| British Thoracic Society | The Thoracic Society of Australia and New Zealand | European Respiratory Society | Spanish Society of Pulmonology | |
|---|---|---|---|---|
| Number of daysAntibiotic treatment in bronchiectasis exacerbation | In general, antibiotic courses for 14 days are standardFurther studies are needed | In patients not requiring parenteral antibiotics for an acute exacerbation, oral antibiotics are prescribed for at least 10-daysFor adult patients failing oral antibiotic therapy, IV antibiotics should be for at least 5-days and often followed by oral antibiotics | Acute exacerbations of bronchiectasis should be treated with 14 days of antibiotics | The guidelines present a division between mild and severe exacerbations:- In mild exacerbations, the guidelines recommends10–21 days (except for: P. aeruginosa, 14–21 days recommended)- In severe exacerbations or with no response to oral treatment or due to resistant microorganisms, the guidelines recommends 14–21 days |
| Eradication treatment following a new isolation of P. aeruginosa or other pathogens | P. aeruginosa eradication recommended as a “good practice point”For patients in whom MRSA is isolated in the sputum, an attempt to eradicate the organism should be made with drug (s), dose and duration guided by local microbiological adviceFor pathogens other than MRSA the topic was not addressed | When P. aeruginosa is first detected, consider discussion with a specialist in this field regarding suitability for eradication. Eradication of other organisms is not addressed | Eradication of first isolation of P. aeruginosa is recommendedThe guidelines suggest not to offer eradication antibiotic treatment to adults with bronchiectasis following new isolation of pathogens other than P. aeruginosa | After a first positive culture for Pseudomonas aeruginosa, treatment to prevent chronic infection is strongly recommendedFor other potentially pathogenic microorganisms, the decision to apply an eradication treatment in initial infection should be made on an individual basis according to the patient's symptoms and the microorganism in question |
| Beginning of long-term antibiotics | Patients having ≥3 exacerbations per year requiring antibiotic therapy or patients with fewer exacerbations that are causing significant morbidity | Macrolides (or other antibiotics) can be considered for therapeutic trial over a limited period (e.g. up to 12–24 months)Considered only in selected patients (e.g. frequent exacerbations: ≥3 exacerbations and/or ≥2 hospitalisations in the previous 12-months) | The guidelines suggest to offer long-term antibiotic treatment for adults with bronchiectasis who have three or more exacerbations per year | The guidelines recommend prolonged antibiotic treatment in all patients who present with chronic bronchial Pseudomonas aeruginosa infectionThe guidelines recommend prolonged antibiotic treatment in all patients with chronic bronchial infection due to other potentially pathogenic microorganisms, who also present ≥2 exacerbations or ≥1 hospitalisation for exacerbation during the previous year, marked decline in lung function or deterioration in quality of life evidenced by an increase in sputum volume or purulence, dyspnoea or coughMacrolides are recommended in patients with clinically stable bronchiectasis but with at least 2 annual exacerbations despite appropriate background treatment |
| Inhaled antibiotics in chronic infection | Patients chronically colonised with Pseudomonas aerugniosa and having ≥3 exacerbations per year requiring antibiotic therapy or patients with fewer exacerbations that are causing significant morbidityThe choice of antibiotic should be guided by the antibiotic sensitivity results | Long-term nebulised antibiotics should not be prescribed routinelyConsider a therapeutic trial in adults with frequent exacerbations and/or Pseudomonas aeruginosa | The guidelines suggest long-term treatment with inhaled antibiotic for adults with bronchiectasis and chronic Pseudomonas aeruginosa infection and who have three or more exacerbations per year | In chronic bronchial infection, inhaled rather than systemic antibiotics are recommended due to their high effectiveness and good safety profileIndication as described above |
A series of bronchiectasis guidelines have been published in recent years – the Spanish Society of Pneumoogy and Thoracic Surgery (SEPAR) guidelines published in 2008 and the British Thoracic Society (BTS) guidelines in 2010 were among the first published guidance for the disease and both have recently been updated.11–13 Outside of Europe there are guidelines from the Thoracic Society of Australia and New Zealand (TSANZ) published in 2015. Most recently the European Respiratory Society guidelines which were published in late 2017.2,14 Each has unique features – the ERS guidelines used the rigorous GRADE methodology and so represent a highly evidence driven synthesis of data, but only addressed 9 key questions around management in adults.2 The TSANZ guidelines are the only recent guidelines to address bronchiectasis in children.14 The guidelines are more comprehensive than the ERS guidelines addressing a large number of clinical questions and use a hybrid evidence method that incorporates systematic review and evidence tables, but permits recommendations based on expert opinion.11 The most recent Spanish guidelines use a Delphi system (a structured method to achieve expert consensus) incorporating some aspects of structured methodology (PICO questions) but provide recommendations on aspects of patient assessment, diagnosis and management which cannot be addressed in a typical evidence based guidelines and as such have the format of a comprehensive disease review article rather than a typical guideline.12,13
It is reassuring that despite the highly diverse methodologies used in putting together these guidelines the resulting recommendations are broadly similar. All guidelines recommend diagnosis based on HRCT chest, testing for underlying causes of bronchiectasis, recommend therapies aimed at improving quality of life and reducing exacerbations, advocate airway clearance techniques and recommend against some ineffective therapies such as recombinant DNAse. Nevertheless, there are differences, some subtle and some more striking, which are worthy of discussion.
What tests should be performed to determine the underlying cause of bronchiectasis? The ERS guidelines recommend immunoglobulins and testing for ABPA should be routine, with additional tests guided by clinical suspicion.2 These tests are prioritised because immunodeficiency and ABPA, along with NTM infection are key treatable causes of bronchiectasis. The TSANZ guidelines are very similar, with the addition of routine sweat testing for children.14 The SEPAR guidelines recommend a highly targeted approach based on clinical features. Culture for bacteria, NTM, fungi, spirometry and immunoglobulin levels are considered routine tests but no other aetiological tests are performed routinely.12 Examples of clinically targeted tests include immunological studies in those with recurrent infections, sweat testing in patients with clinical features of CF, ABPA testing in those with central bronchiectasis and oesophageal manometry in patients with gastroesophageal reflux. The absence of routine testing for ABPA may represent a perception that it is less common in Southern vs Northern Europe. Testing for Alpha-1 antitrypsin routinely remains controversial with no large studies to determine whether this is or is not useful in patients without typical features such as emphysema. Routine testing is not included in the BTS or ERS guidelines for this reason.
Long term antibiotic treatment remains highly controversial, particularly in view of recent unsuccessful phase 3 trials.7,8,15 The SEPAR guidelines take a highly aggressive approach, recommending that all patients with Pseudomonas aeruginosa infection should receive inhalational antibiotics regardless of severity or history of exacerbations, while recommending this treatment also for patients without P. aeruginosa and a history of 2 or more exacerbations despite standard therapy. In this case, it is important to point out that these recommendations are at odds with published evidence. The recently published RESPIRE trials investigated patients with and without P. aeruginosa and a history of 2 or more exacerbations and found no clear evidence of benefit of inhaled antibiotics.7,8,15 Likewise the ORBIT studies which included only patients with P. aeruginosa, while showing an overall pooled benefit in terms of reduced exacerbations found no quality of life or lung function benefit of inhaled antibiotics, consistent with previous reports.15 Therefore the existing evidence and prognostic studies do not support the routine use of inhaled antibiotics for patients without frequent exacerbations.16 Inhaled antibotics present a high burden to users because of the time involved with their administration which adds to the need to reserve them for patients most likely to benefit. As a result, the ERS, BTS and TSANZ guidelines are united in recommending reserving inhaled antibiotics for patients with 3 or more exacerbations per year. Other less striking differences are discussed in the table below. The optimal duration of antibiotic treatment for an exacerbation is not known and so several guidelines recommend 10–14 day courses as standard, while up to 21 days is considered in the SEPAR guidance.2,13 Eradication of P. aeruginosa is another area of significant controversy where the majority of evidence is from retrospective case series. Despite the low level of evidence, and based primarily on experience in CF, the ERS, SEPAR and BTS guidelines recommend eradication of P. aeruginosa at first isolation using various regimes. The TSANZ guidelines are more reticent, suggesting to seek specialist advice.14 Several guidelines also suggest possible eradication attempts for MRSA, but only the SEPAR guidelines suggest the possibility to eradicate organisms other than P. aeruginosa, an area where evidence is entirely lacking.13
We are in a period where the bronchiectasis evidence base is evolving, and guidelines internationally differ in how far they are willing to go “beyond the evidence” to provide guidance to clinicians. So how do we put evidence into practice? We would suggest that for clinicians who do not specialise in bronchiectasis care, the priority is “getting the basics right”. All guidelines agree that patients should be diagnosed by HRCT, tested for immunodeficiency and ABPA, should be taught airway clearance techniques and receive prompt antibiotic treatment of exacerbations with a minimum of 10 days antibiotics. Implementation of these measures universally would represent a major advance for BE patients worldwide.
For more specialist issues, we need more knowledge and more trials, but in the absence of consistent evidence there will remain differences in practice between specialists, centres and countries in the use of therapies such as eradication, inhaled antibiotics and mucoactive drugs. Such differences are understandable and in our view guidelines should avoid being overly prescriptive by making “strong” recommendations where “strong” evidence is lacking.
With a view to future guidelines, what serves patients best? Expert advice that is not based on evidence? Or evidence based recommendations that ignore important topics because there is not enough evidence?1 Ultimately, guidelines must provide what clinicians and patients are looking for and so a balance should be sought that makes guidelines as practical as possible while maintaining methodological rigour.
FundingEuropean Respiratory Society through the EMBARC network. JDC is supported by the GSK/BLF Chair of Respiratory Research.
Conflicts of InterestJDC is chair of the European Bronchiectasis Guidelines 2017 and is a member of the British Thoracic Society bronchiectasis guideline committee. MR reports no conflicts of interest.