Asthma is a well-established respiratory disease characterized by airway narrowing and inflammation. Until recently, the foundation of asthma management recommended by the Global Initiative for Asthma (GINA), consisted of the use of short acting beta-agonists (SABA), to which further therapy is added. SABAs rapidly relieve airway smooth muscle contraction leading to improvement in airflow obstruction, and thereby, asthma related symptoms. SABAs were previously recommended as the sole agent on an as-needed basis in those with mild disease. SABAs, however, fail to address the inflammatory component of asthma. The inflammatory pattern in asthma is predominantly eosinophilic and thus patients, at a minimum those with more than mild disease, require the addition of inhaled corticosteroids (ICS) for maintenance therapy. Thus, concern was raised as to the potential adverse effects of the use of beta-agonist bronchodilators, both short acting (SABA) and long-acting (LABA), without concomitant ICS.
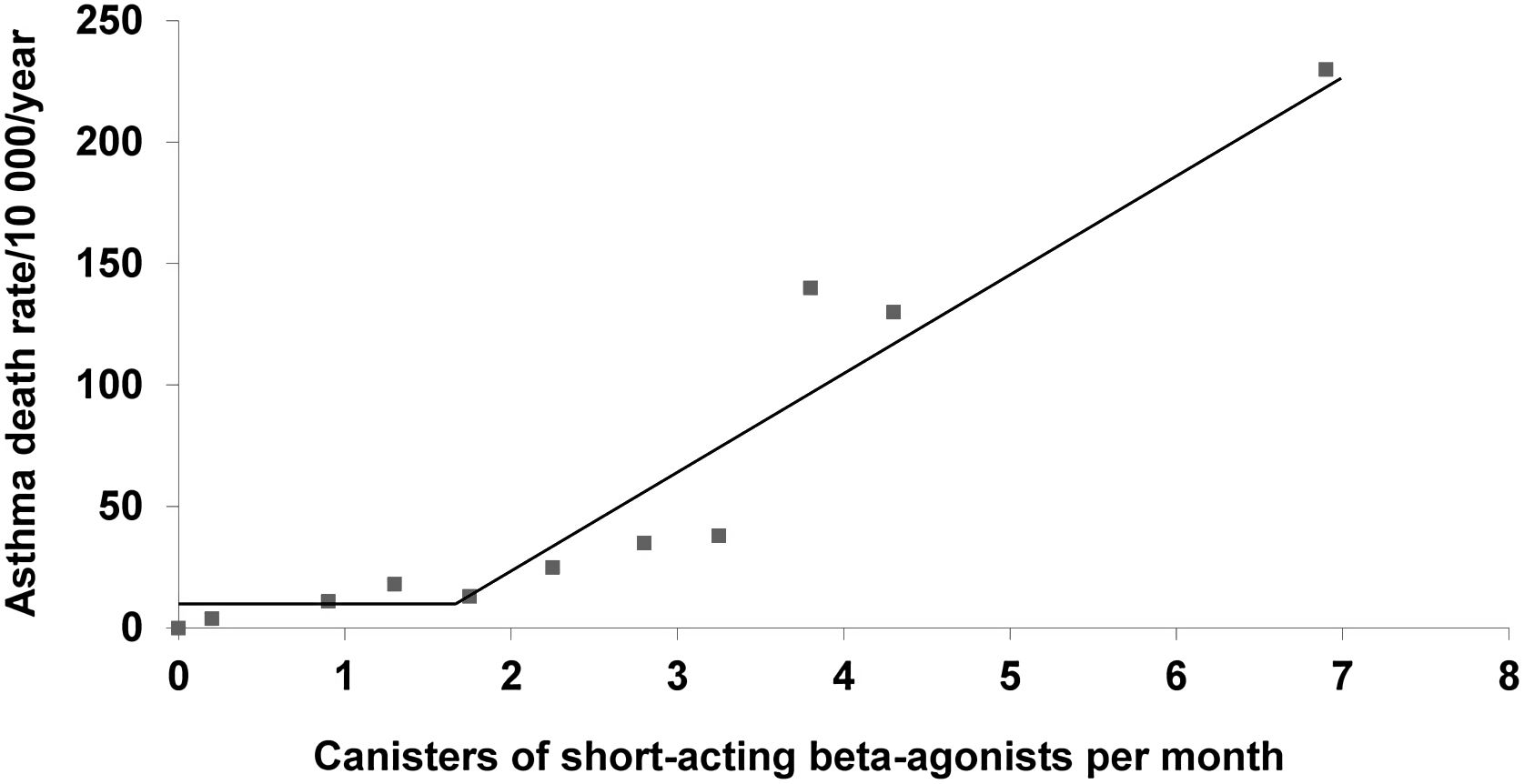
The dangers of SABA overuse in asthma were established more than 25 years ago.1 In a large population-based cohort study of patients dispensed asthma medications, inhaled SABAs were associated with a dose–response increase in deaths from asthma, especially if used in excess of 1.7 canisters per month (Fig. 1). Worsening bronchial hyperresponsiveness with the regular use of a SABA was proposed as a mechanism.2 However, a further population-based cohort study showed substantial reduced risk of asthma deaths with regular use of ICS suggesting that under utilisation of ICS was the more likely underlying cause of the association between asthma death and the overuse of SABAs.3
Asthma death rate as a function of the number of SABA inhalers per month from a large population-based cohort study of patients treated for asthma, with change-point analysis indicating the sharp increase at 1.7 canisters per month (adapted from Figure 4 of Suissa et al.1).
More recent studies reported similar findings. In the global SABA use in asthma (SABINA) study, a cohort of 365,324 patients with a diagnosis of asthma, aged 12–45 years, were stratified according to number of SABA canister purchases in the baseline 12-month period.4 Over a mean 85 months of follow-up, SABA use during the baseline year was associated with increased risks of all-cause and asthma-related mortality in a dose-dependent manner (hazard ratios 2.3; 95% CI 2.0–2.7 and 31.7; 95% CI 11.9–84.7), respectively, for patients dispensed 11 or more SABA canisters during the baseline year compared to those using 2 or less, regardless of other concomitant asthma treatment at baseline. This association, however, must be interpreted with caution as it is also consistent with confounding by disease severity and the data analysis did not account for concurrent ICS use.
With the introduction of long-acting beta-agonists for the treatment of asthma, questions as to their safety as sole controller therapy for asthma were raised. In a meta-analysis that compiled data from 19 randomized placebo-controlled trials, totalling 33,826 patients with asthma, LABA use without the addition of an inhaled corticosteroid (ICS), increased severe and life-threatening exacerbations, as well as asthma related deaths, compared to placebo.5 A separate review of trials where LABA was given as an add-on therapy, suggested that the combination of ICS and LABA was not associated with adverse outcomes as compared to ICS only controller therapy.6 This was further studied in four FDA-mandated multicenter, randomized, double-blind trials where asthma patients with a history of severe exacerbations were assigned to receive an ICS or an ICS and LABA combination, and the data pooled.7 While dual therapies were associated with fewer asthma exacerbations, the question of asthma-related death remained unresolved, with 2 versus 0 asthma-related deaths in the dual and ICS only treatment arms, respectively, across the trials (rate ratio 3.0; 95% CI: 0.2–37.7).8 These large trials suggest that long-acting beta-agonists have an important role in asthma control, but not as monotherapy. These findings have been reflected in the GINA recommendations for the treatment of moderate asthma for many years now. The use of LABA without ICS is postulated to obscure the symptoms of eosinophilic airway inflammation, allowing it to go unchecked, resulting in increased morbidity and mortality. Nonetheless, the issue of asthma-related mortality with LABA added to ICS, though extremely rare, persists.8
While the predominant inflammatory profile seen in asthma is eosinophilic, which is the most corticosteroid responsive, asthma is now recognized to be a heterogenous disease. With recent advances in biomarkers such as blood and sputum eosinophils, and airway fractional exhaled nitric oxide (FeNO), a non-eosinophilic asthma (NEA) endotype has been identified and can represent a sizable subgroup of patients.9,10 Unlike eosinophilic asthma, these patients do not improve with corticosteroids. When treated with a 10–14-day regimen of oral and inhaled corticosteroids, patients with ICS-naïve eosinophilic asthma improved their airflow obstruction by 8.6%, compared to −0.2% for those with ICS-naïve NEA.10 However, both groups responded similarly and significantly to SABA even after corticosteroid therapy. There is also evidence to suggest that patients with NEA can lower or stop pre-existing ICS therapy without worsened clinical outcomes.11
These studies imply that there exists a sub-population of asthmatics who might benefit from beta-agonist bronchodilators without corticosteroid therapy, though this may be an oversimplification. These studies have all been small, with no more than 80 carefully selected patients in each study, and their real-world applicability is limited. In contrast, the studies evaluating adverse events with SABA and LABA monotherapy are much larger and have better generalizability. Furthermore, the NEA endotype may not be stable. When followed longitudinally for 6 months with repeat sputum cytology, 40% of patients with steroid naive NEA treated with LABA only, developed transient sputum eosinophilia.12 It is particularly concerning that the patients who developed this airway eosinophilia on LABA did not report more symptoms or demonstrate deterioration by spirometry, which is in keeping with the theory that bronchodilators may mask underlying inflammation. This inflammation, or lack thereof, can only be reliably evaluated using sputum cytology which has limited availability. Blood eosinophils and FeNO, albeit helpful, are imperfect surrogate markers.13
In practical terms, outside of specialized centers, access to these biomarkers is currently very limited and most patients with asthma are mild and are followed in primary care. Therefore, even if there exist patients who may do well on bronchodilators alone, they will be difficult to identify and follow. Thus, currently the best approach for most patients is the introduction of an ICS at asthma diagnosis, even if asthma is considered as mild, and to avoid beta-agonist monotherapy. When evaluated head-to-head, as needed ICS-formoterol combination therapy was found to be superior to as needed SABA in asthma symptom control and reduced asthma exacerbations by almost two-thirds.14 Moreover, it was also non-inferior to daily maintenance ICS therapy in preventing asthma exacerbations.15 These findings essentially render SABAs obsolete except in the acute care situation, as they can be replaced completely in GINA Steps 1 and 2 by ICS-formoterol. This is reflected in the GINA guidelines as of 201916 – where a SABA is now no longer recommended as the first step therapy for mild asthma. Based on the existing data, the move away from SABAs will result in better asthma control for more patients, while avoiding potential excessive use associated with increased asthma-related mortality. However, the heterogeneity of asthma is becoming better characterized and is already challenging previously established principles. As more data emerges, the role of beta-agonist bronchodilators may need to be re-examined.
Conflict of InterestS. Suissa attended scientific advisory committee meetings or received speaking fees from AstraZeneca, Atara, Boehringer-Ingelheim, Bristol-Myers-Squibb, Merck, Novartis, Panalgo, Pfizer and Seqirus. N. Zhao and P. Ernst have no conflict of interest to declare.