Combination inhaled corticosteroid (ICS)/fast-onset beta2-agonist reliever therapy is based on the concept of titrating the ICS dose through the vehicle of bronchodilator reliever use, and represents the optimal method to respond to day-to-day changes in airways inflammation in asthma. This therapeutic approach has now been investigated with both ICS/formoterol and ICS/salbutamol reliever treatments, across the range of asthma severity, primarily in adolescents and adults, and shown to reduce the risk of severe exacerbations compared with otherwise comparable regimens based on short-acting beta2-agonist (SABA) reliever therapy.1–8
The main initial focus of this research has been on ICS/formoterol prescribed according to the maintenance and reliever therapy (MART) regimen in moderate and severe asthma. When compared with maintenance ICS/long acting beta2-agonist (LABA) plus SABA reliever therapy, MART reduces the risk of severe exacerbations (and associated oral corticosteroid exposure) by about one third, reduces airways inflammation, avoids the requirement for multiple inhalers, prevents SABA monotherapy in those non-compliant with their maintenance ICS/LABA treatment, and reduces episodes of beta2-agonist overuse leading to delay in seeking medical review which is a common contributing cause of death from asthma.2,5,6,9
More recently, focus has turned to ICS/formoterol reliever alone, without the requirement for maintenance ICS-based treatment, in mild asthma. This approach represents an alternative to both SABA reliever therapy alone and maintenance ICS plus SABA reliever therapy, and has been the subject of a recent European Respiratory Society (ERS) Task Force review.10
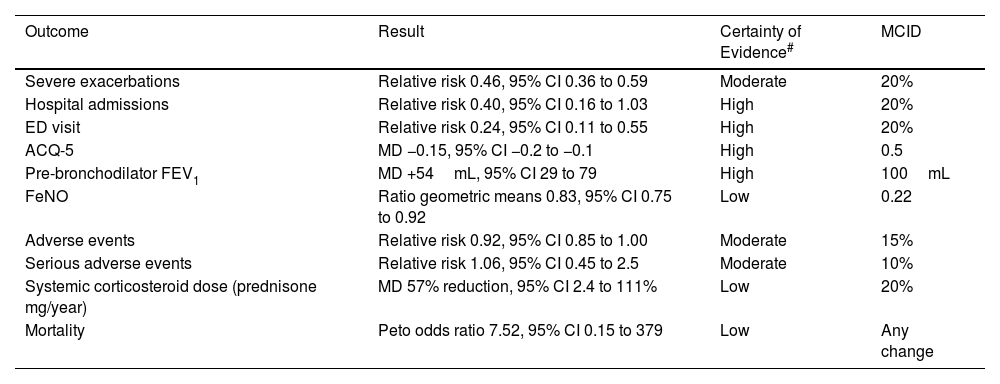
ICS/Formoterol Reliever vs SABA Reliever Therapy in Mild Asthma (Table 1a)The main therapeutic advantages of ICS/formoterol vs SABA reliever therapy are the greater than 50% reduction in severe exacerbation risk, together with the reduction in severe exacerbations resulting in an Emergency Department visit of about 75%.10 This regimen also results in an improvement in asthma control and lung function although this is less than the minimal clinically important difference (MCID). It results in a reduction in fractional expired nitric oxide (FeNO), a marker of airways inflammation, thereby leading to its designation as anti-inflammatory reliever (AIR) therapy. The exposure to ICS is low due to infrequent reliever use in this population, and is offset to some degree by the reduced oral corticosteroid exposure, and thus there is unlikely to be any clinically relevant difference in overall systemic corticosteroid exposure.
ICS–Formoterol Reliever Therapy vs SABA Reliever Therapy: Efficacy and Safety.
| Outcome | Result | Certainty of Evidence# | MCID |
|---|---|---|---|
| Severe exacerbations | Relative risk 0.46, 95% CI 0.36 to 0.59 | Moderate | 20% |
| Hospital admissions | Relative risk 0.40, 95% CI 0.16 to 1.03 | High | 20% |
| ED visit | Relative risk 0.24, 95% CI 0.11 to 0.55 | High | 20% |
| ACQ-5 | MD −0.15, 95% CI −0.2 to −0.1 | High | 0.5 |
| Pre-bronchodilator FEV1 | MD +54mL, 95% CI 29 to 79 | High | 100mL |
| FeNO | Ratio geometric means 0.83, 95% CI 0.75 to 0.92 | Low | 0.22 |
| Adverse events | Relative risk 0.92, 95% CI 0.85 to 1.00 | Moderate | 15% |
| Serious adverse events | Relative risk 1.06, 95% CI 0.45 to 2.5 | Moderate | 10% |
| Systemic corticosteroid dose (prednisone mg/year) | MD 57% reduction, 95% CI 2.4 to 111% | Low | 20% |
| Mortality | Peto odds ratio 7.52, 95% CI 0.15 to 379 | Low | Any change |
CI: confidence interval; ED: Emergency Department; ACQ-5: Asthma Control Questionnaire – 5; FEV1: forced expiratory volume in 1 second; FeNO: fractioned exhaled nitric oxide; MCID: minimal clinically important difference; MD: mean difference. Data taken from European Respiratory Society Task Force (Ref. 10).
GRADE Working Group grades of evidence: high certainty: confident that the true effect lies close to that of the estimate of the effect; moderate certainty: moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; very low certainty: little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
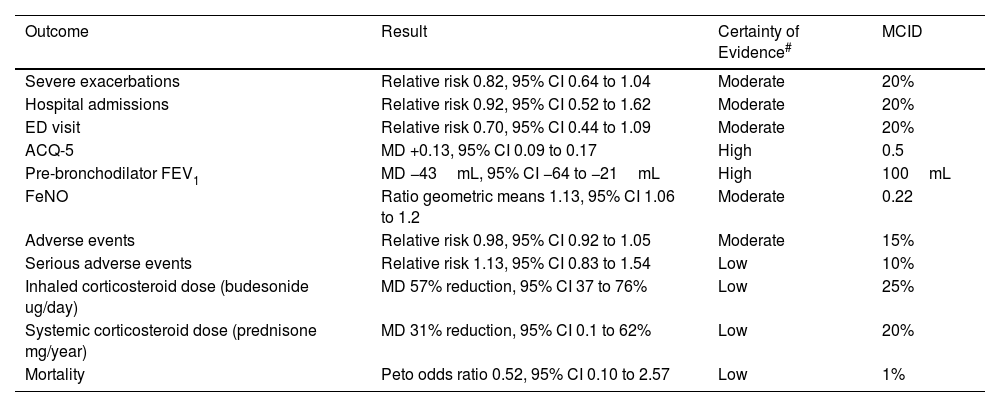
ICS/formoterol reliever alone results in a non-significant reduction in the risk of severe exacerbations vs ICS maintenance plus SABA reliever therapy, and severe exacerbations resulting in an Emergency Department visit.10 These findings may overestimate the efficacy of maintenance ICS, as adherence to regular scheduled ICS treatment is likely to be worse in routine clinical practice than in clinical trials. Asthma control, lung function, asthma-associated quality of life and FeNO favour maintenance ICS, but the differences are small and less than the MCID.
ICS–Formoterol Reliever Therapy vs ICS Maintenance Plus SABA Reliever Therapy: Efficacy and Safety.
| Outcome | Result | Certainty of Evidence# | MCID |
|---|---|---|---|
| Severe exacerbations | Relative risk 0.82, 95% CI 0.64 to 1.04 | Moderate | 20% |
| Hospital admissions | Relative risk 0.92, 95% CI 0.52 to 1.62 | Moderate | 20% |
| ED visit | Relative risk 0.70, 95% CI 0.44 to 1.09 | Moderate | 20% |
| ACQ-5 | MD +0.13, 95% CI 0.09 to 0.17 | High | 0.5 |
| Pre-bronchodilator FEV1 | MD −43mL, 95% CI −64 to −21mL | High | 100mL |
| FeNO | Ratio geometric means 1.13, 95% CI 1.06 to 1.2 | Moderate | 0.22 |
| Adverse events | Relative risk 0.98, 95% CI 0.92 to 1.05 | Moderate | 15% |
| Serious adverse events | Relative risk 1.13, 95% CI 0.83 to 1.54 | Low | 10% |
| Inhaled corticosteroid dose (budesonide ug/day) | MD 57% reduction, 95% CI 37 to 76% | Low | 25% |
| Systemic corticosteroid dose (prednisone mg/year) | MD 31% reduction, 95% CI 0.1 to 62% | Low | 20% |
| Mortality | Peto odds ratio 0.52, 95% CI 0.10 to 2.57 | Low | 1% |
CI: confidence interval; ED: Emergency Department; ACQ-5: Asthma Control Questionnaire – 5; FEV1: forced expiratory volume in 1 second; FeNO: fractioned exhaled nitric oxide; MCID: minimal clinically important difference; MD: mean difference. Data taken from European Respiratory Society Task Force (Ref. 10).
GRADE Working Group grades of evidence: high certainty: confident that the true effect lies close to that of the estimate of the effect; moderate certainty: moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; very low certainty: little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
ICS/formoterol reliever reduces both ICS and oral corticosteroid exposure, likely leading to reduced associated systemic adverse effects with long term use. The greater than 50% reduction in ICS exposure with ICS/formoterol is important as it suggests that the timing of the ICS dose, when delivered through the vehicle of reliever use, is a determinant of efficacy, as well as the total daily ICS dose. This is consistent with available data which indicates that ICS/formoterol has greater potency and efficacy when administered as a reliever than as regular scheduled maintenance treatment.11
There is a strong patient preference for ICS/formoterol reliever, with 90% of participants in a clinical trial who had taken ICS/formoterol for 12 months expressing a preference to continue with this regimen, rather than transfer over to maintenance ICS plus SABA reliever therapy.12 This preference is likely due to the simplicity of the regimen, with the requirement to only take one inhaler as needed, rather than two inhalers one of which needed to be taken twice every day regardless of symptoms.
GeneralisabilityGeneralisability of the above evidence is limited in a number of respects. Firstly, there have been no studies in children under the age of 12, which means that the findings relate only to adolescents and adults. Secondly, the evidence has been obtained from randomised controlled trials of the budesonide/formoterol turbuhaler dry powder inhaler (DPI), and not from other combination products or devices.
ERS Task Force RecommendationsThe European Respiratory Society established a Task Force to provide evidence-based recommendations on the use of as-needed ICS/formoterol as treatment for mild asthma, using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.10 The Task Force recommended that adult and adolescent patients with mild asthma use as-needed ICS/formoterol instead of as-needed SABA (strong recommendation; low certainty of evidence). This recommendation was based on the marked benefit of as-needed ICS/formoterol in mild asthma on severe exacerbation outcomes and the risks related to as-needed SABA in the absence of anti-inflammatory treatment. In accordance with the GRADE approach used, the designated low certainty of evidence was based on the lowest grading of any of the prespecified critical outcomes, which in this case was mortality risk, for which an assessment of risk was severely limited due to the rarity of this outcome.
The Task Force suggests that adults with mild asthma use as-needed ICS/formoterol instead of regular ICS maintenance treatment plus as-needed SABAs, and that adolescents with mild asthma use either as-needed ICS/formoterol or ICS maintenance treatment plus as-needed SABA (conditional recommendation; low certainty of evidence).10 The recommendation for adults places a relatively higher value on the reduction of systemic corticosteroid use and the outcomes related to exacerbations, and a relatively lower value on the small differences in asthma control. Either treatment option was suggested for adolescent patients as the balance was very close and data more limited.
The FutureObtaining evidence in children is a priority, and the findings of a number of ongoing randomised controlled trials are awaited with interest. The efficacy and safety of ICS/formoterol in the treatment of severe exacerbations in the Emergency Department is another priority as high dose ICS have efficacy in this setting and there is the potential that if ICS/formoterol is administered in equipotent beta2-agonist doses as SABA treatment, its use will lead to greater efficacy.13 Finally, there is preliminary evidence that as-needed ICS/salbutamol alone has efficacy in mild asthma,7 and further research is required to better understand its place in asthma management.
The implementation of ICS/formoterol is limited in many countries, including the European Union, due to lack of regulatory approval. In countries in which ICS/formoterol is available other barriers include cost, and reluctance of doctors and patients to change the entrenched clinical practice of prescribing SABAs alone or together with ICS-based treatment.
ConclusionThe GINA recommendation that ICS/formoterol reliever therapy is preferred rather than SABA reliever therapy across the range of asthma severity can be considered a paradigm change in the management of asthma.1 The evidence indicating the efficacy/safety profile of as-needed ICS/formoterol in mild asthma has contributed to this recommendation.
Conflict of InterestRB, DF and AP are members of the ERS Task Force Report ‘Short guidelines for the use of as-needed ICS/formoterol in mild asthma’. RB has received institutional research funding from AstraZeneca, Genentech, Health Research Council of New Zealand and CureKids (NZ); personal fees from AstraZeneca, Avillion, Cipla and Teva; AP has received institutional research funding from Chiesi, AstraZeneca, GlaxoSmithKline and Sanofi; personal fees from Chiesi, AstraZeneca, GlaxoSmithKline, Menarini, Novartis, Zambon, Mundipharma, Sanofi, Edmond Pharma, Iqvia, Avillion, Regeneron, Elpen Pharmaceuticals, Moderna and Roche. DF has no conflicts of interest to declare.