Early detection is crucial to improve lung cancer survival rates. Delays in diagnosis might negatively impact the prognosis of the disease. This study aims to analyze the diagnostic delay in lung cancer patients and describe if there is an association between delay and survival.
MethodsThe data source used was the Thoracic Tumor Registry of the Spanish Lung Cancer Group. This analysis was restricted to lung cancer cases with information on the first date of consultation by symptoms and date of diagnosis. The delay was calculated as the number of days between the two dates. A descriptive analysis was performed, and ordinal logistic regressions were fitted with delay as the dependent variable. Kaplan–Meier survival analysis and Cox regression were performed.
Results22,755 lung cancer cases were included. Never smokers were 1.16 (95%CI: 1.06–1.27) times more likely to register longer delay than smokers. Stage 0–I–II cases had a 3.09 (95%CI: 2.88–3.32) higher risk of longer delay compared to III–IV stages. Overall, 5-year survival rate after diagnosis was 23.64% (95%CI: 22.88–24.41). In those categorized as having the shortest delay 5-year survival was 17.67% (95%CI: 16.31–19.07) and in the extreme delay it was 32.98% (95%CI: 31.28–34.69) (p<0.001). Adjusted mortality risk was higher in those with the shortest delay (HR 1.36, CI95%: 1.30–1.43) in comparison with the extreme delay.
ConclusionsDiagnostic delay is short among Spanish lung cancer patients, indicating a relatively quick diagnostic process. Extreme delays appear to be associated with higher survival rates, possibly attributed to slow-growing tumors, earlier stage at diagnosis or basically the natural history of this disease.
Lung cancer is a major public health challenge worldwide. This neoplasm is one of the main causes of morbidity and mortality, with a constantly increasing incidence and a relatively low survival rate compared to other cancer types,1 despite improvements in diagnosis and treatment. This may be related to late identification and delayed diagnosis of the disease.
Several strategies have been developed and implemented for the early detection of lung cancer with the ultimate goal of improving its prognosis. The most important strategy in this regard is the implementation of a lung cancer screening program. Several studies have been conducted to prove its effectiveness in reducing lung cancer mortality. In Europe, the NELSON study, the largest in terms of sample size, showed a 26% (95%CI 9–41%) reduction in lung cancer mortality after 10 years of follow-up in men.2 However, in many of the smaller studies conducted in Europe, screening appears to be less effective in terms of early detection. In addition, the risk-benefit ratio has been questioned in health technology assessment reports3 and, in Spain, only 63.5% of subjects with lung cancer would be eligible for screening applying the 2021 United States Preventive Service Task Force (USPSTF) criteria.4 This percentage is lower for women compared to men (47.5% vs 70.0%, respectively).4
In this regard, an alternative strategy to improve lung cancer survival is the early detection of the disease in primary care, minimizing the diagnostic delay.5 The delay in diagnosis of lung cancer can be attributed to a variety of reasons, including a late onset of symptoms6 or the presence of general and non-specific symptoms such as cough and dyspnea.7–10 These clinical manifestations are often attributed to other common diseases and comorbidities the patients have,11 leading to under-diagnosis and delayed specialist consultation. In addition, patients generally take a long time to consult a primary care physician about their symptoms, due to their low awareness of the risk of lung cancer or poor access to healthcare.12 Moreover, the fear of being diagnosed with lung cancer, especially among smokers, can lead patients to deny their symptoms and delay seeking medical care.9,13 In this context, it is crucial to understand the factors that may be linked to diagnostic delay in lung cancer patients and the potential consequences that this may have in terms of survival. Identifying and addressing diagnostic delay may improve early detection and ultimately improve clinical outcomes. Therefore, this study aims to analyze the diagnostic delay in lung cancer patients in Spain, examine the characteristics of cases associated with long delay and describe potential associations between delay and lung cancer survival.
It is important to mention that Spain has a universal healthcare coverage. When a patient has symptoms related with chest diseases (i.e. dyspnea, cough, etc.), they go to the corresponding primary care physician. Each Spaniard has one assigned. This physician might ask for lab tests or simple imaging (i.e. chest Rx), following the clinical interview. If the patient signs, symptoms and results are compatible to lung cancer, the patient enters in a so-called fast clinical pathway and is derived to a chest physician, ending in diagnosis if lung cancer is present.
MethodsStudy design and data sourceA cross-sectional study was conducted using the Spanish Lung Cancer Group Thoracic Tumor Registry (Registro de Tumores Torácicos-RTT) as the main data source.
The Thoracic Tumor Registry is a monographic registry of thoracic cancer cases diagnosed in Spain. The methodology of the registry has been described in detail elsewhere.14 In summary, the RTT included its first participant in 2016 and currently more than 80 Spanish hospitals contribute with thoracic cancer cases to the registry. Recently, the RTT's representativeness by sex and age of lung cancer cases diagnosed in Spain was confirmed.15
The RTT is registered in ClinicalTrials.gov (NCT02942458), and the study protocol has been approved by the institutional committee of the Puerta de Hierro University Teaching Hospital (Majadahonda, Madrid) (no. PI 148/15).
Data collectionThe last access to the registry's database was on 30 January 2024. This study therefore included lung cancer cases diagnosed and registered up to that date.
For analysis purposes, we included all patients diagnosed with lung cancer that had information on the first date of consultation for lung cancer symptoms (or when the patient went to the emergency room as a consequence of undiagnosed lung cancer) and date of diagnosis. The date of diagnosis was the date of a positive biopsy confirming lung cancer. The diagnostic delay (hereinafter, delay) was calculated as the number of days between the two dates and categorized by quartile as follows: shortest delay 1–10 days, short delay 11–23 days, long delay 24–53 days and extreme delay>53 days. For the survival analysis, the patient's status (alive, dead, or lost to follow-up) was collected at each follow-up visit after diagnosis. In the event of death, the date of death was recorded.
Additionally, the following variables were collected from lung cancer cases included in the study: age, sex (male, female, other), tobacco use (never smoker, ex-smoker, smoker), number of pack-years (in the case of smokers and ex-smokers), main occupation, exposure to second-hand smoke in the last 20 years at home (defined as have been living with a smoker in the last 20 years), histological type (adenocarcinoma, adenosquamous cell carcinoma, squamous cell carcinoma, large-cell carcinoma, sarcomatoid carcinoma, NOS/undifferentiated, small-cell carcinoma, carcinoid, thymic carcinoma and others), stage at diagnosis (categorized as 0–I–II, III–IV, SCLC limited, SCLC extended and other), presence of comorbidities at diagnosis (yes, no) and presence of symptoms at diagnosis (yes, no).
Statistical analysisFirst, a descriptive analysis was performed, describing the absolute and relative frequency of the included cases according to the variables of interest (namely, age, sex, tobacco use, histologic type, stage at diagnosis, exposure to second-hand smoke, main occupation and presence of comorbidities and symptoms at diagnosis). In addition, the delay was calculated as median and interquartile range (IR) according to the aforementioned variables.
Second, we conducted a bivariate analysis comparing delays according to the previously mentioned variables of interest using crude logistic regressions in which the dependent variable was delay categorized into four categories (shortest delay 1–10 days, short delay 11–23 days, long delay 24–53 days and extreme delay>53 days). Ordinal logistic regressions were then fitted, as the categories of the dependent variable (delay) follow a logical order. Shortest delay was the reference category. In this analysis, age, sex, tobacco use, stage at diagnosis, main occupation and the presence of comorbidities and symptoms were included as independent variables. Odds ratios (OR) were calculated with their 95% confidence intervals (95%CI).
Third, Kaplan–Meier survival analysis was performed to assess the time to death of the included cases. Censored times (i.e. where no death is recorded during follow-up) were included in the analysis. The survival function was calculated, and the probability of survival was estimated annually for the first 5 years after diagnosis with their 95%CI. In addition, the survival function was calculated according to stage at diagnosis, sex and delay using two categories (shortest and extreme delay). The log-rank test was used to compare survival curves. Additionally, a Cox regression analysis was performed to identify the independent variables related to survival. In this analysis, delay (categorized), age, sex, stage at diagnosis and tobacco use were included as independent variables. Hazard Ratios (HR) were calculated along with their 95%CI. Sensitivity analyses were performed considering the “waiting time paradox” by excluding lung cancer cases diagnosed within 7 days and 28 days of the first symptoms.16
Statistical analysis was performed with Stata v.17. Statistical significance was set at p-value<0.05.
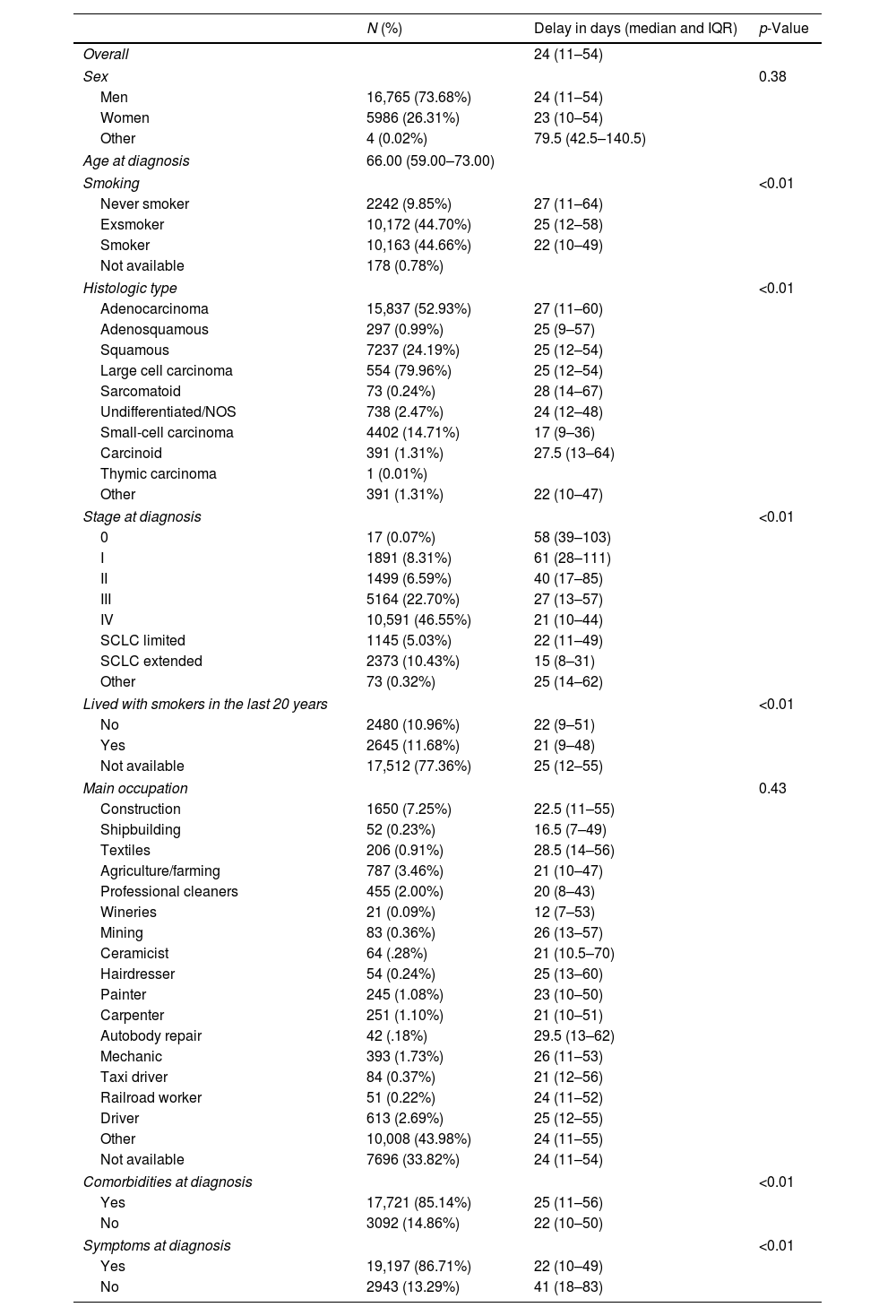
ResultsIn total, 22,755 lung cancer cases were included. Most were men (73.68%), the median age at diagnosis was 66 years (interquartile range 59–73), and most were smokers (44.66%) and ex-smokers (44.70%). Table 1 shows the main characteristics of the included cases and the median days of delay, broken down by the characteristics of interest. Overall, the median number of days of delay was 24 (IQR 11–54). The median number of days of delay was greater in never smokers (27 days, IQR 11–64) than in smokers (22 days, IQR 10–49), in subjects with stage 0 and I at diagnosis compared to subjects with more advanced stages (Table 1), in some occupations such as textile workers (28.5 days, IQR 14–56) or autobody repair workers (29.5 days, IQR 13–62), and in subjects without symptoms at diagnosis (41 days, IQR 18–83) compared to those with symptoms (22 days, IR 10–49).
Main characteristics of the included lung cancer cases, expressed as absolute and relative frequency, and the median days of delay, by variables of interest.
| N (%) | Delay in days (median and IQR) | p-Value | |
|---|---|---|---|
| Overall | 24 (11–54) | ||
| Sex | 0.38 | ||
| Men | 16,765 (73.68%) | 24 (11–54) | |
| Women | 5986 (26.31%) | 23 (10–54) | |
| Other | 4 (0.02%) | 79.5 (42.5–140.5) | |
| Age at diagnosis | 66.00 (59.00–73.00) | ||
| Smoking | <0.01 | ||
| Never smoker | 2242 (9.85%) | 27 (11–64) | |
| Exsmoker | 10,172 (44.70%) | 25 (12–58) | |
| Smoker | 10,163 (44.66%) | 22 (10–49) | |
| Not available | 178 (0.78%) | ||
| Histologic type | <0.01 | ||
| Adenocarcinoma | 15,837 (52.93%) | 27 (11–60) | |
| Adenosquamous | 297 (0.99%) | 25 (9–57) | |
| Squamous | 7237 (24.19%) | 25 (12–54) | |
| Large cell carcinoma | 554 (79.96%) | 25 (12–54) | |
| Sarcomatoid | 73 (0.24%) | 28 (14–67) | |
| Undifferentiated/NOS | 738 (2.47%) | 24 (12–48) | |
| Small-cell carcinoma | 4402 (14.71%) | 17 (9–36) | |
| Carcinoid | 391 (1.31%) | 27.5 (13–64) | |
| Thymic carcinoma | 1 (0.01%) | ||
| Other | 391 (1.31%) | 22 (10–47) | |
| Stage at diagnosis | <0.01 | ||
| 0 | 17 (0.07%) | 58 (39–103) | |
| I | 1891 (8.31%) | 61 (28–111) | |
| II | 1499 (6.59%) | 40 (17–85) | |
| III | 5164 (22.70%) | 27 (13–57) | |
| IV | 10,591 (46.55%) | 21 (10–44) | |
| SCLC limited | 1145 (5.03%) | 22 (11–49) | |
| SCLC extended | 2373 (10.43%) | 15 (8–31) | |
| Other | 73 (0.32%) | 25 (14–62) | |
| Lived with smokers in the last 20 years | <0.01 | ||
| No | 2480 (10.96%) | 22 (9–51) | |
| Yes | 2645 (11.68%) | 21 (9–48) | |
| Not available | 17,512 (77.36%) | 25 (12–55) | |
| Main occupation | 0.43 | ||
| Construction | 1650 (7.25%) | 22.5 (11–55) | |
| Shipbuilding | 52 (0.23%) | 16.5 (7–49) | |
| Textiles | 206 (0.91%) | 28.5 (14–56) | |
| Agriculture/farming | 787 (3.46%) | 21 (10–47) | |
| Professional cleaners | 455 (2.00%) | 20 (8–43) | |
| Wineries | 21 (0.09%) | 12 (7–53) | |
| Mining | 83 (0.36%) | 26 (13–57) | |
| Ceramicist | 64 (.28%) | 21 (10.5–70) | |
| Hairdresser | 54 (0.24%) | 25 (13–60) | |
| Painter | 245 (1.08%) | 23 (10–50) | |
| Carpenter | 251 (1.10%) | 21 (10–51) | |
| Autobody repair | 42 (.18%) | 29.5 (13–62) | |
| Mechanic | 393 (1.73%) | 26 (11–53) | |
| Taxi driver | 84 (0.37%) | 21 (12–56) | |
| Railroad worker | 51 (0.22%) | 24 (11–52) | |
| Driver | 613 (2.69%) | 25 (12–55) | |
| Other | 10,008 (43.98%) | 24 (11–55) | |
| Not available | 7696 (33.82%) | 24 (11–54) | |
| Comorbidities at diagnosis | <0.01 | ||
| Yes | 17,721 (85.14%) | 25 (11–56) | |
| No | 3092 (14.86%) | 22 (10–50) | |
| Symptoms at diagnosis | <0.01 | ||
| Yes | 19,197 (86.71%) | 22 (10–49) | |
| No | 2943 (13.29%) | 41 (18–83) | |
Missing data. Age: 349; histologic type: 1; stage: 2; lived with smokers: 118; main occupation: 70; comorbidities: 1942; symptoms: 615.
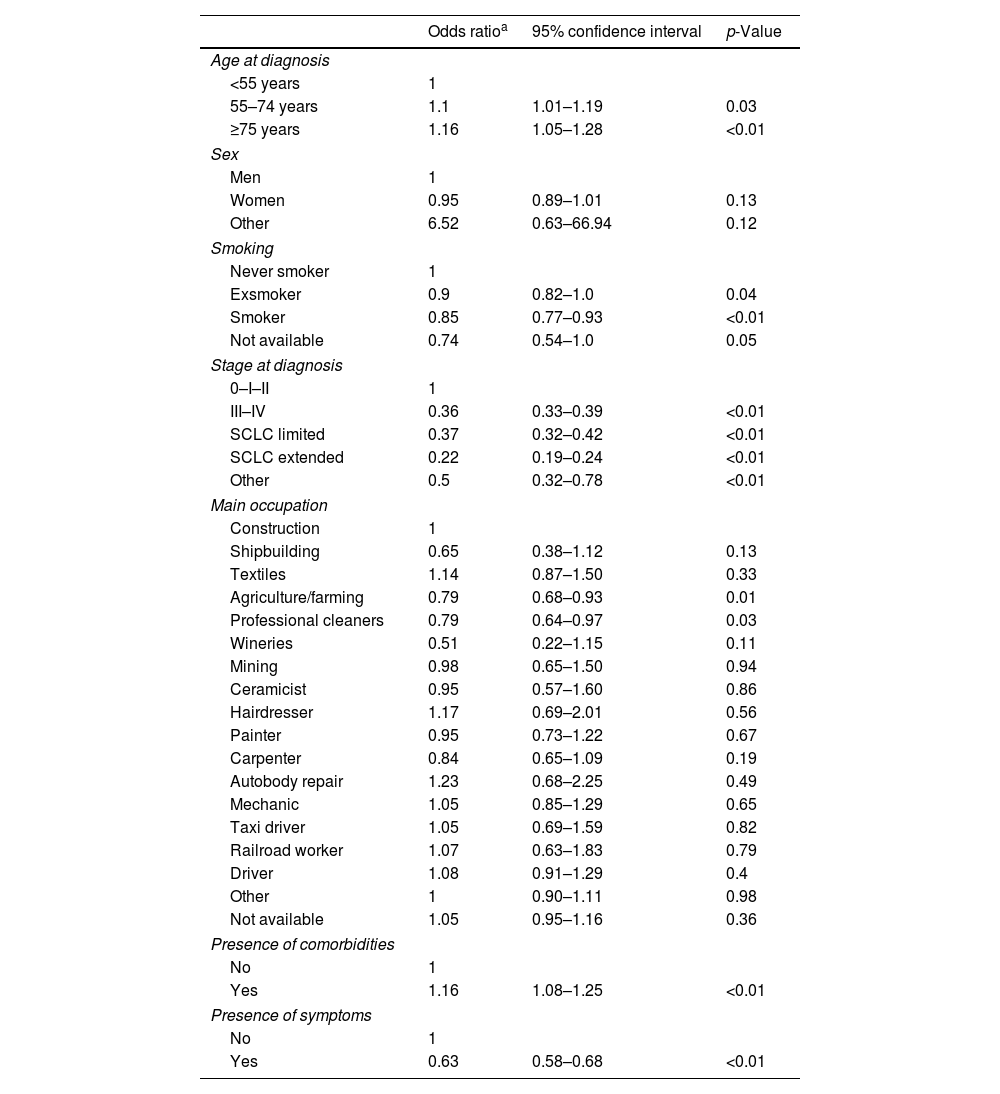
Bivariate analysis using delay categorized in four categories showed a significant association between delay and smoking, stage at diagnosis and age at diagnosis (data not shown). Multivariate analysis (Table 2) showed that older adults were more likely to have longer delay than those aged less than 55 years old at the time of diagnosis and smokers were less likely to have longer delay than never-smokers.
Multivariate ordinal regression for analyzing predictors on diagnostic delay.
| Odds ratioa | 95% confidence interval | p-Value | |
|---|---|---|---|
| Age at diagnosis | |||
| <55 years | 1 | ||
| 55–74 years | 1.1 | 1.01–1.19 | 0.03 |
| ≥75 years | 1.16 | 1.05–1.28 | <0.01 |
| Sex | |||
| Men | 1 | ||
| Women | 0.95 | 0.89–1.01 | 0.13 |
| Other | 6.52 | 0.63–66.94 | 0.12 |
| Smoking | |||
| Never smoker | 1 | ||
| Exsmoker | 0.9 | 0.82–1.0 | 0.04 |
| Smoker | 0.85 | 0.77–0.93 | <0.01 |
| Not available | 0.74 | 0.54–1.0 | 0.05 |
| Stage at diagnosis | |||
| 0–I–II | 1 | ||
| III–IV | 0.36 | 0.33–0.39 | <0.01 |
| SCLC limited | 0.37 | 0.32–0.42 | <0.01 |
| SCLC extended | 0.22 | 0.19–0.24 | <0.01 |
| Other | 0.5 | 0.32–0.78 | <0.01 |
| Main occupation | |||
| Construction | 1 | ||
| Shipbuilding | 0.65 | 0.38–1.12 | 0.13 |
| Textiles | 1.14 | 0.87–1.50 | 0.33 |
| Agriculture/farming | 0.79 | 0.68–0.93 | 0.01 |
| Professional cleaners | 0.79 | 0.64–0.97 | 0.03 |
| Wineries | 0.51 | 0.22–1.15 | 0.11 |
| Mining | 0.98 | 0.65–1.50 | 0.94 |
| Ceramicist | 0.95 | 0.57–1.60 | 0.86 |
| Hairdresser | 1.17 | 0.69–2.01 | 0.56 |
| Painter | 0.95 | 0.73–1.22 | 0.67 |
| Carpenter | 0.84 | 0.65–1.09 | 0.19 |
| Autobody repair | 1.23 | 0.68–2.25 | 0.49 |
| Mechanic | 1.05 | 0.85–1.29 | 0.65 |
| Taxi driver | 1.05 | 0.69–1.59 | 0.82 |
| Railroad worker | 1.07 | 0.63–1.83 | 0.79 |
| Driver | 1.08 | 0.91–1.29 | 0.4 |
| Other | 1 | 0.90–1.11 | 0.98 |
| Not available | 1.05 | 0.95–1.16 | 0.36 |
| Presence of comorbidities | |||
| No | 1 | ||
| Yes | 1.16 | 1.08–1.25 | <0.01 |
| Presence of symptoms | |||
| No | 1 | ||
| Yes | 0.63 | 0.58–0.68 | <0.01 |
a The regression was adjusted for all variables shown in the table.
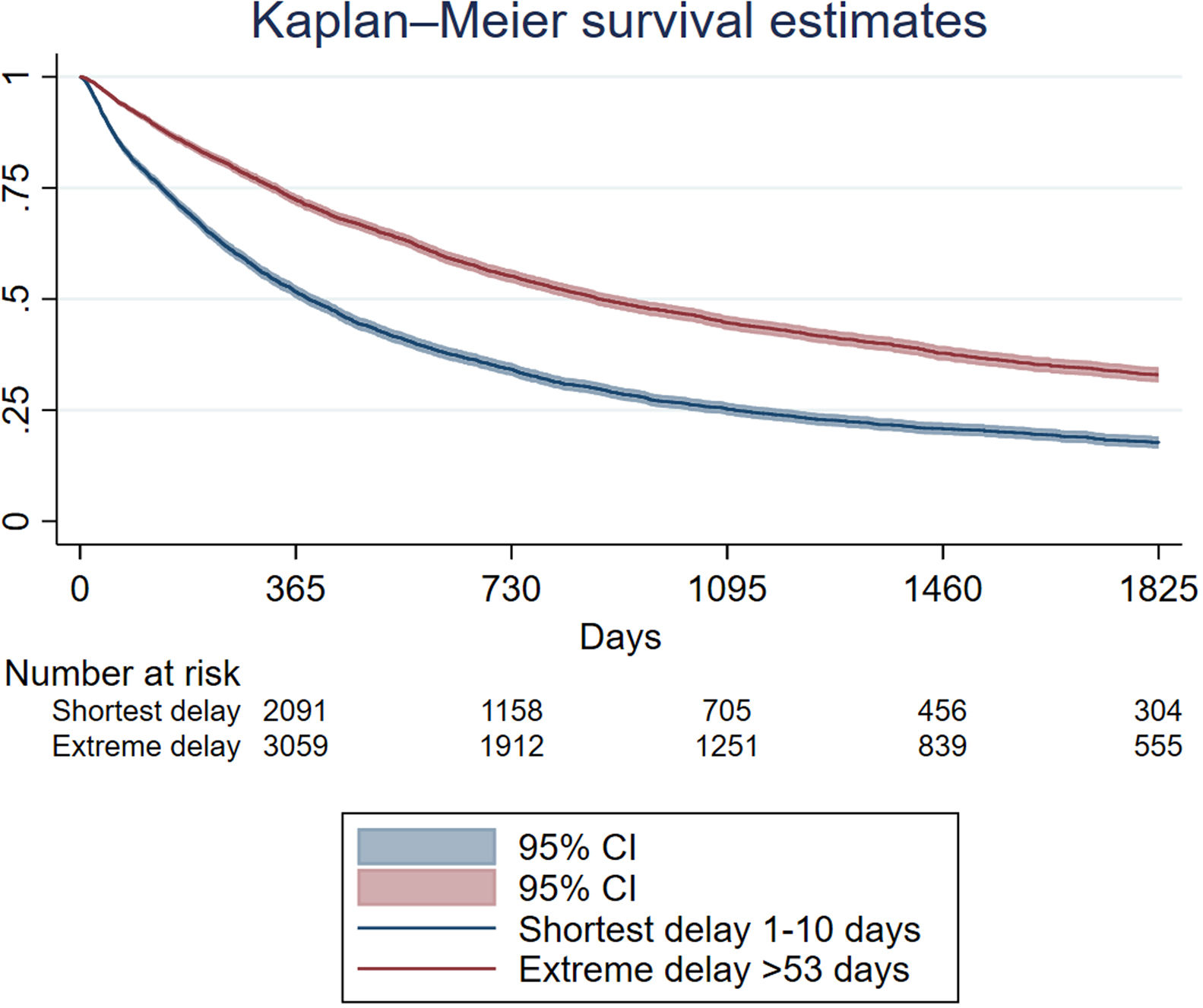
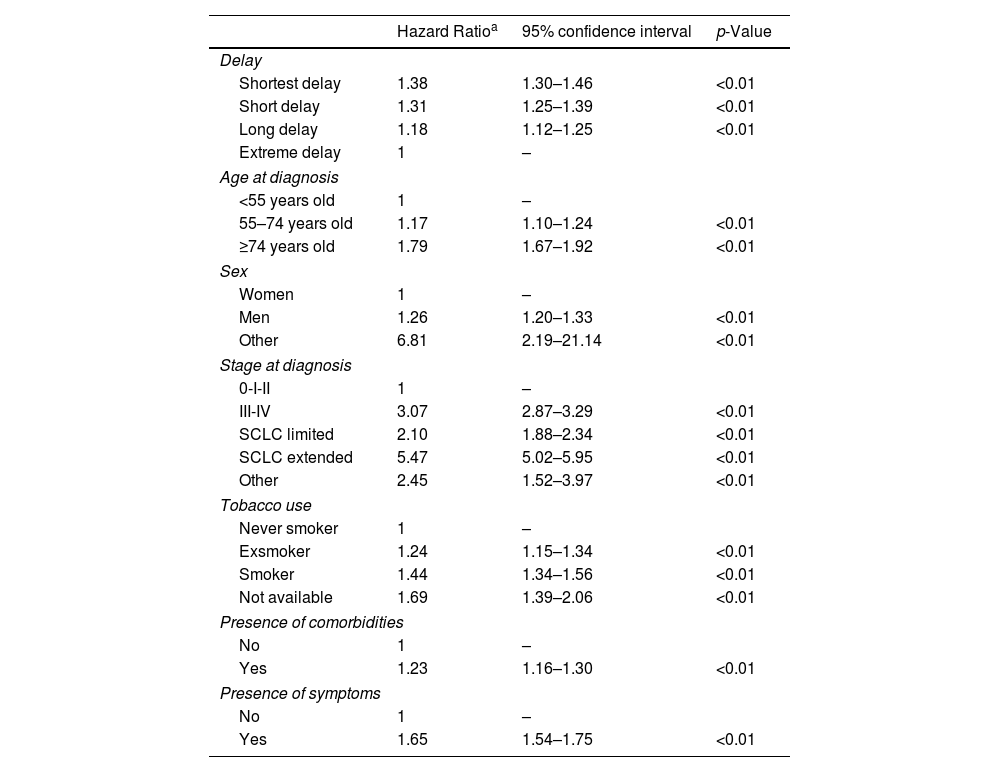
The mean follow-up time was 623 days after diagnosis, 839 days for those who did not present the event and 484 for those who presented the event (death). Overall, the one-year survival rate after diagnosis was 59.34% (95%CI 58.63–60.05), while the 5-year survival rate was 23.64% (95%CI 22.88–24.41). In Supplementary Table 1, the annual overall survival for the first 5 years after diagnosis by sex and delay is described. In those categorized as having the shortest delay, 5-year survival was 17.67% (95%CI 16.31–19.07) and in those categorized as having extreme delay, it was 32.98% (95%CI 31.28–34.69) (p<0.005). Fig. 1 shows the survival estimates of the cases with shortest delay and extreme delay. Women have higher survival than men at any point in time (Supplementary Table 1).
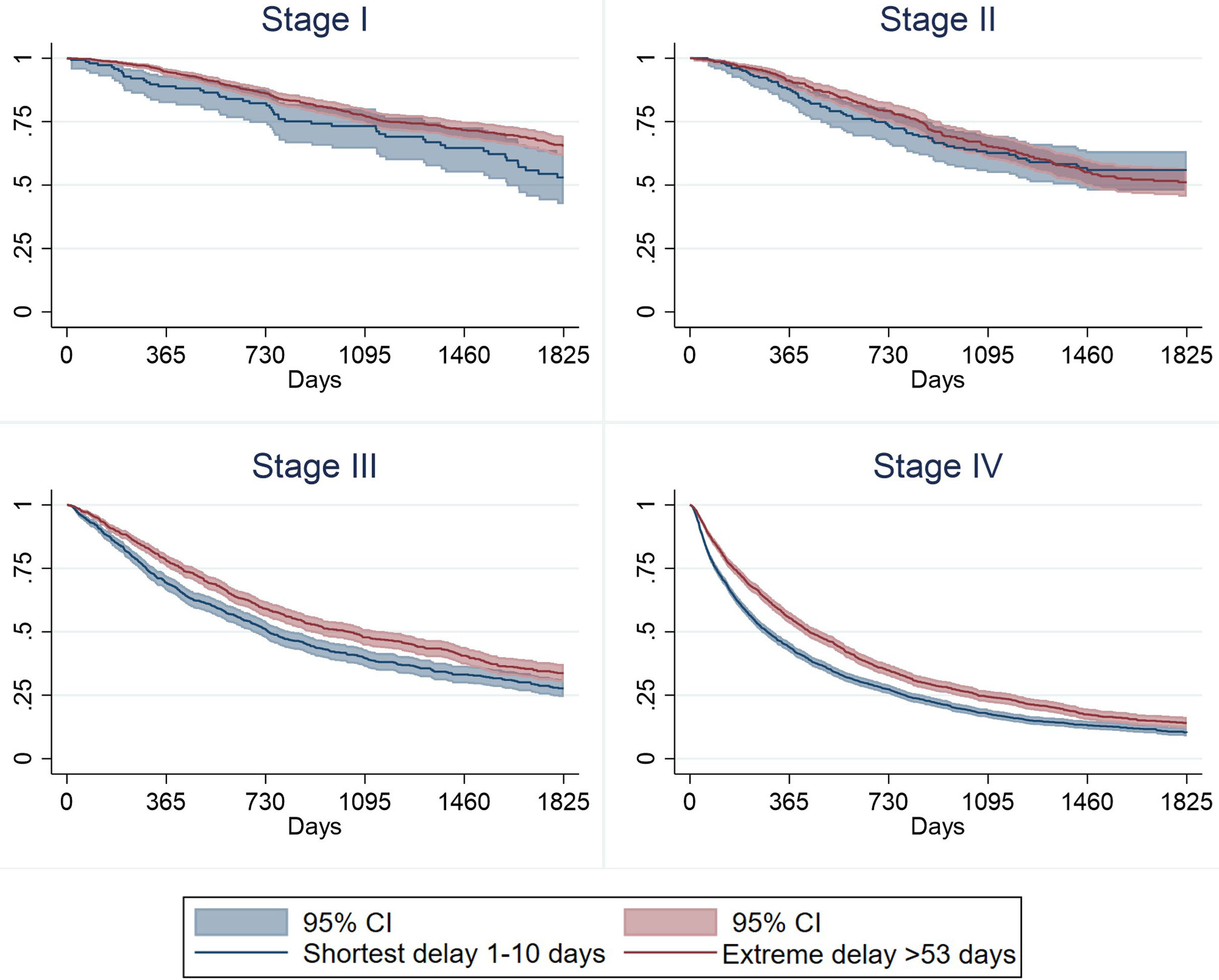
Fig. 2 shows survival estimates by both delay and stage at diagnosis (restricted to stage I, II, III and IV). It is observed that although survival is always higher in those with extreme delay, independently of the stage at diagnosis, when the stage at diagnosis is more advanced, differences in survival between shortest delay and extreme delay become more evident (Table 3).
Cox regression analysis of variables predicting the overall survival.
| Hazard Ratioa | 95% confidence interval | p-Value | |
|---|---|---|---|
| Delay | |||
| Shortest delay | 1.38 | 1.30–1.46 | <0.01 |
| Short delay | 1.31 | 1.25–1.39 | <0.01 |
| Long delay | 1.18 | 1.12–1.25 | <0.01 |
| Extreme delay | 1 | – | |
| Age at diagnosis | |||
| <55 years old | 1 | – | |
| 55–74 years old | 1.17 | 1.10–1.24 | <0.01 |
| ≥74 years old | 1.79 | 1.67–1.92 | <0.01 |
| Sex | |||
| Women | 1 | – | |
| Men | 1.26 | 1.20–1.33 | <0.01 |
| Other | 6.81 | 2.19–21.14 | <0.01 |
| Stage at diagnosis | |||
| 0-I-II | 1 | – | |
| III-IV | 3.07 | 2.87–3.29 | <0.01 |
| SCLC limited | 2.10 | 1.88–2.34 | <0.01 |
| SCLC extended | 5.47 | 5.02–5.95 | <0.01 |
| Other | 2.45 | 1.52–3.97 | <0.01 |
| Tobacco use | |||
| Never smoker | 1 | – | |
| Exsmoker | 1.24 | 1.15–1.34 | <0.01 |
| Smoker | 1.44 | 1.34–1.56 | <0.01 |
| Not available | 1.69 | 1.39–2.06 | <0.01 |
| Presence of comorbidities | |||
| No | 1 | – | |
| Yes | 1.23 | 1.16–1.30 | <0.01 |
| Presence of symptoms | |||
| No | 1 | – | |
| Yes | 1.65 | 1.54–1.75 | <0.01 |
a The regression was adjusted for all variables shown in the table and occupation (not shown in the table).
The Cox regression showed a mortality risk higher in men (adjusted Hazard Ratio (HR) 1.26; 95%CI 1.20–1.33), aged 74 or more years old (HR 1.79; 95%CI 1.67–1.92), in stages III and IV (HR 3.07; 95%CI 2.87–3.29) and in ever-smokers (exsmokers HR 1.24, 95%CI 1.15–1.34; smokers HR 1.44, 95%CI 1.34–1.56). The mortality risk was higher in those with the shortest delay (HR 1.38, 95%CI 1.30–1.46) in comparison with extreme delay. Sensitivity analyses, which exclude those cases diagnosed within the subsequent 28 days (n=12,373) and 7 days (n=3,536) following the onset of symptoms, are described in Supplementary Tables 2 and 3 and Supplementary Fig. 1 and show no relevant differences.
DiscussionThis study highlights various questions related to lung cancer survival related with diagnostic delay. The first one is that, in Spain, diagnostic delay can be considered, in general, short, and lung cancer patients may benefit from a quick diagnosis leading to receive the most adequate treatment as soon as possible since Spain has a public and free national health system. The second one is that there are various aspects related with a longer delay at diagnosis, such as tobacco use, stage at diagnosis, and age at diagnosis. It is observed that the subjects with the longest delay are those with the longest survival, even accounting for the “waiting time paradox”.
The median number of days from symptom onset to diagnosis in our study was 24 days. In some countries, such as the United Kingdom, recommendations on waiting times for lung cancer diagnosis and treatment were established. However, there is currently low adherence to these standards.10 Although the delay is relatively brief in Spain, it still does not comply with the established targets (the UK recommendation is 14 days to diagnosis). The results of our study align with those of previous research conducted in other countries.17 We also observed a shorter delay compared to other studies.18,19 A systematic review20 of articles published up to November 2020 revealed that the median/mean waiting time from symptoms to diagnosis was more than 20 days in the included studies. Furthermore, it was observed that in those studies that specified the stage at diagnosis, the delay was shorter than in those that did not. Similarly, the median delay for small cell lung cancer was slightly shorter (69 days) than that for non-small cell lung cancer (75 days), and this aligns with our results. Other research showed a shorter interval between the onset of symptoms and diagnosis in comparison with our study.21
There is a direct association with diagnostic delay and the presence of symptoms at diagnosis. Those presenting with symptoms take half of the time to be diagnosed compared with patients with no symptoms (22 vs 41 days). This expected result is also related with the shortest delay presented by patients in late stages (i.e. Stage IV take 21 days to be diagnosed vs 61 days for Stage I patients). These results are in line with the previous literature and might mean that symptoms’ presentation and the clinical presentation of the patient speed up diagnosis. Furthermore, Walter et al. identified an association between presenting chest/shoulder pain and shorter diagnostic delays in lung cancer patients. In the adjusted model, it was observed that presenting hemoptysis was the only symptom that could predict lung cancer diagnosis, but it was not common among cases as the first symptom.7
We have observed that smoking status appears to be associated with time to diagnosis, with smokers being diagnosed earlier than never-smokers, with 5 days of difference. This has to do with the pre-test probability of lung cancer of a never-smoker, where primary care practitioners and neumologists do not think on a never smoker presenting with lung cancer and the patient might be derived to cardiovascular tests if he or she presents dyspnea.
In concordance with previous studies,22 we have not observed a difference on diagnostic delay by sex, and this is positive, because, in Spain, lung cancer is more frequent in men compared to women. We were expecting a shorter delay for men, but this lack of difference could mean that clinicians and particularly primary health care practitioners might be aware that lung cancer is increasingly being presented in Spanish women. It is surprising that those which have not defined their sex show a greater delay. No other studies have reported this result so far. A qualitative study involving patients with non-binary gender reported that these patients often have negative experiences in healthcare settings. This may affect their attendance at consultations when they present with symptoms.23
The association between diagnostic delay and survival has been studied extensively in recent years, with the hypothesis that longer delays in diagnosis decrease survival, and some studies have so concluded.16,24–26 However, most published studies conclude the opposite20 and even associate longer diagnostic delays with better outcomes in terms of survival and mortality.19,27–29 Most of the articles linking a short diagnostic delay with worse prognosis justified this relationship by stating that patients were in more advanced stages, or were older or presented more comorbidities, and therefore were referred and treated more quickly than those diagnosed in earlier stages. In these more advanced patients, the poor prognosis did not change despite the faster treatment, resulting in shorter survival. This is evidenced by the study conducted by Romine et al.,30 which found that when the analysis was restricted to cases that died within six months of diagnosis, the association between higher delay and lower survival was no longer observed. This situation can be exemplified with the relatively frequent situation of a patient presenting an extended small cell lung cancer whose first contact with the health system is through the emergency room and is being diagnosed very soon after. This situation shows a short diagnostic delay but a very poor survival and is reflected when we observe that small cell lung cancer has the shortest diagnostic delay (17 days) compared with adenocarcinoma (27 days).
Previous literature indicates that one of the primary reasons for delay in seeking medical care is related to patients’ lack of risk perception or anxiety when consulting a physician.31 Various strategies have been developed with the aim of increasing public awareness in this regard. For example, Kennedy et al.32 conducted a campaign for early diagnosis of lung cancer in the United Kingdom, using chest X-rays. The campaign demonstrated a notable shift toward earlier stages of lung cancer, with a 9.3% reduction in patients diagnosed at advanced stages of the disease. Other studies33–35 have also reached positive conclusions, indicating that interventions aimed at increasing public awareness are effective in terms of increasing the number of consultations for respiratory symptoms, without increasing anxiety or cancer-related concerns. Despite these findings, uncertainty persists regarding the efficacy of these interventions. Indeed, several of them have not demonstrated efficacy. Furthermore, the cost of these campaigns is considerable, estimated at approximately £23,000 per case of lung cancer detected.5,36,37
The main limitation of this study is that the date on which the individual consulted for symptoms was used to calculate the delay, rather than the date on which the symptoms started. The estimated delay would be greater if the second date were available for analysis. A further limitation is that in some cases some symptoms might have been underreported or misreported by the patient to the referring clinician, but we have no way to assess this. We recognize the limitation that the RTT does not include patient factors such as health literacy and socioeconomic status, which could provide further insights into diagnostic delays and survival. Furthermore, there may still be unmeasured confounding factors affecting our results, despite our efforts to control for some key variables, such as comorbidities. Although it is not possible to rule out lead-time bias, in our study its potential influence is considered limited because there is no lung cancer screening program in place in Spain.
The principal strength of this study lies in the large sample size, which allows for the categorization of the delay into four categories, thereby ensuring more accurate results. A further and important strength is that we have the information to measure the impact not only in stage at diagnosis but also on overall survival of these patients, an information which is not always available in these studies. Finally, it is important to highlight the representativeness of the RTT of the lung cancer cases diagnoses in Spain,15 which enables the results to be generalized to the country level.
Our findings have implications for clinical practice and policy. The contradiction in our results, where greater diagnostic delay appears to be associated with longer survival, may be attributed to limitations related to waiting times. Guirado et al.20 reported that patients diagnosed at earlier stages do not experience accelerated waiting times compared to those diagnosed at later stages, where urgency is higher due to the high mortality rate of lung cancer. Therefore, health systems should prioritize reducing diagnostic delays by optimizing referral pathways and ensuring timely access to diagnostics, particularly for high-risk populations. Public education campaigns regarding lung cancer symptoms and the importance of early medical consultation are critical for reducing delays, as supported by previous research.32 Further investigation is necessary to understand the factors contributing to diagnostic delays and to evaluate interventions aimed at mitigating them.
To conclude, diagnostic delays are generally short among Spanish lung cancer patients, indicating a relatively quick diagnostic process. This study identified several characteristics related with a longer delay at diagnosis, such as tobacco use, stage at diagnosis, and age at diagnosis. Additionally, extreme delays appear to be associated with higher survival rates, possibly attributed to slow-growing tumors or early-stage at diagnosis.
Ethics approvalThe Thoracic Tumors Registry was registered in ClinicalTrials.gov (NCT02942458), and the study protocol was approved by the institutional committee of the Puerta de Hierro University Teaching Hospital (Majadahonda, Madrid) (no. PI 148/15).
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributorship statementARR: Conceptualization, Methodology, Supervision, Writing-review and editing.
MP: Conceptualization, Methodology, Supervision, Writing-review and editing.
CCP: Methodology, Data curation, Formal analysis, Visualization, Writing-original draft.
The rest of the authors: Investigation, Resources, Writing-review and editing.
Conflicts of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
Artificial intelligence involvementThe authors declare no artificial intelligence software of tool was used in this manuscript.
None.