Swimmers have larger lungs and higher diffusion capacity than other athletes and the general population,1,2 but there is no clear evidence whether genetic predisposition, swimming training, or a mixture of both factors account for these changes. During incremental exercise, lung diffusion capacity for carbon monoxide (DLCO) increases in a linear fashion with cardiac output following the distension of capillaries that are already perfused and/or the recruitment of additional capillaries that are not perfused under resting conditions3 but DLCO also has been decreased after exercise in some cases.4,5 To the best of our knowledge there are no information about possible changes in the lung's alveoli capillary interface after swimming training, although swimming-induced pulmonary oedema (SIPO) has been reported in the literature, as a specific lung injury related to swimming.6,7
We evaluate the possible alterations in the pulmonary alveolar-capillary diffusion induced by swimming exercise before and after 10 training sessions over a 4-weeks training phase.
The participants were 21 junior elite swimmers, including 7 females and 14 males [16.1±1.6 years, 21.6±1.3 body mass index, 57.7±3.63 VO2max, 137.7±18.6 VEmax, 110±10 FVC (%-predicted), 108±10 FEV1 (%-predicted)]. Measurements of DLCO were performed pre-training and post-training along 10 swimming training sessions, conducted every 2–3 days, over a period of 4 weeks, with the objective of evaluating the full range of swimming training sessions faced by the swimmers, in terms of intensity, volume, and schedule. The measurements were taken less than 10min pre- and post-training by a computerized spirometer (Ganshorn, PowerCube Diffusion+, Niederlauer, Germany) by means of the single-breath method and DLCO was corrected to individual haemoglobin (Hb) concentration at the beginning of the study. Differences in DLCO from pre-, to post-training were analyzed using a two-way repeated measures analysis of variance (2w-RM ANOVA) and statistical effects were established at p<0.05. All procedures were in accordance with the ethical standards of the Clinical Research Ethics Committee at the Direcció General de l’Esport of the Catalonian Sports Council.
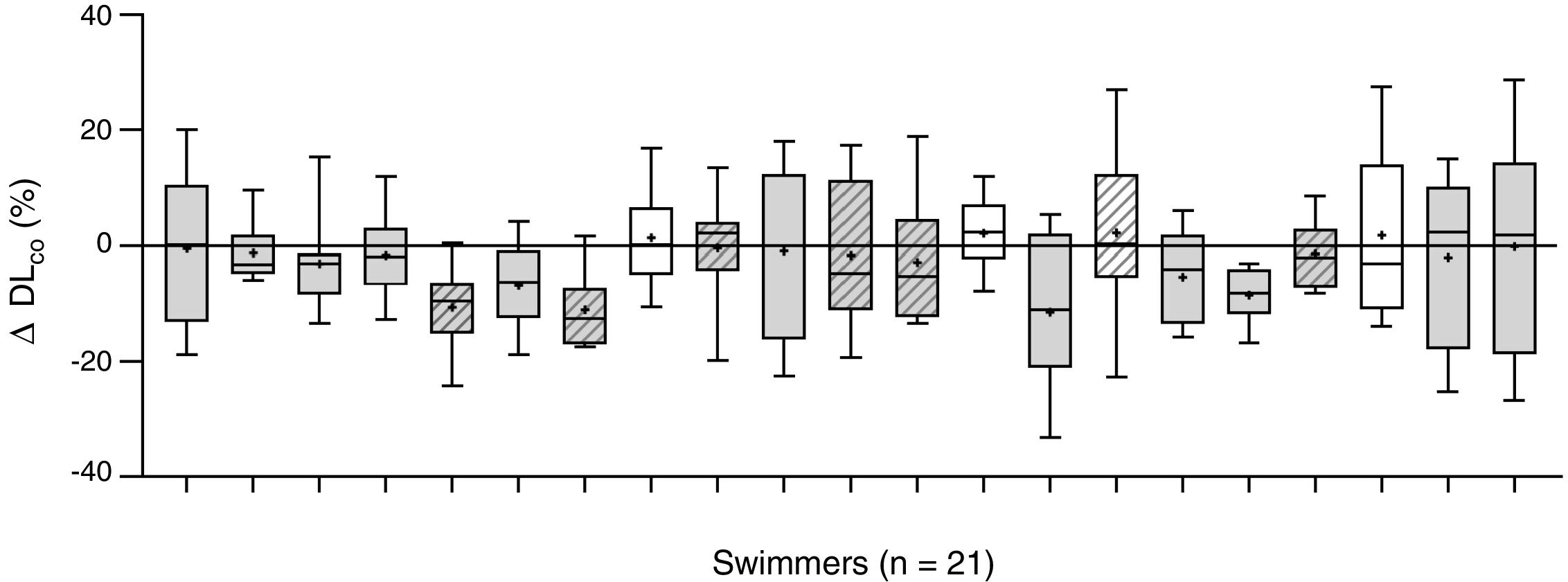
The changes of the lung diffusing capacity and lung volume along the follow-up period are described in Fig. 1, comparing the changes (%) between averaged pre-training DLCO and the averaged post-training DLCO along the 10 training sessions. There was a significant interaction between changes in DLCO and training (F=17.32, p=0.001, ŋ2p=0.55), with a slight decrease in lung diffusion capacity for carbon monoxide (DLCO) after swimming exercise during the follow-up (44.4±8.1 to 43.3±8.9mLmin−1mmHg−1, p=0.047; Fig. 1).
Intra-individual changes in DLCO after swimming exercise. Comparison of the pre-training DLCO versus the post-training DLCO along the 10 training sessions in the 21 participants. Individual data is showed as a box plot (average decrease, in grey; and average increase, in white). Mean (+), and median (line) values are also represented. Female swimmers are represented by diagonal lines inside their box plot and male swimmers with not-dashed box plots.
Swimmers from this study had large alveolar volumes (125±15%) and extremely developed basal lung diffusing capacity (DLCO) (151±21%). The exact mechanism behind the improved lung volume and diffusing capacity in elite swimmers remains unclear. The respiratory mechanics of swimming comprise a rapid out-of-water phase of forced inhalation and a relaxed underwater phase of prolonged exhalation, which must be coordinated with the stroke mechanics.1 This condition modifies the respiratory mechanics towards a low respiratory frequency and a high tidal volume pattern which lead to increases in work of breathing8 and inspiratory muscle strength.9 Possible metabolic pathways related with hypoxia-activated genes and mechanical stress in the alveoli capillaries are also related with lung growth10 suggesting that either repeated pulmonary expansion to TLC,11 or repeated exposure to apnoeic periods10 during swimming could stimulate lung growth in swimmers. A longitudinal study or a twin study should be performed to fully elucidate the influence of swimming training in lung function.
To the best of our knowledge, this is the first report assessing the changes of lung diffusion capacity in relation to swimming training. We show a consistent slight decrease in DLCO (−2.5%) after swimming exercise, along 207 pre- to post-comparative evaluations. Some studies have already shown that lung diffusion is reduced after exercise in land-based athletes4,5 leading to the speculation that exercise could trigger interstitial pulmonary oedema, but the novelty of this case report is the follow-up analysis of swimming, an aquatic-based sport specifically associated to pulmonary oedema.6,7 The most important finding is the large inter-individual variability in the response of DLCO to swim training along the follow-up. Fig. 1 shows individual change in DLCO post-training. We show a decrement in averaged DLCO (x¯DLCO) in 17 of 21 participants along the 10 training sessions evaluated. Large inter-individual differences in x¯DLCO are also showed after swimming exercise, including 6 subjects showing a large decrease (−5.6–11.2%), 11 subjects with a small decrease (−0.4–3.1%) and 4 subjects showing a slight increase (+1.3–2.2%). Therefore, at least 6 of 21 participants of the study suffer a post-training diffusion limitation consistently more pronounced than the repeatability of DLCO in healthy adults (±3.1%).12
Several causes has been proposed to explain this decrease, including the redistribution of central blood volume to peripheral areas13 and the development of an exercise-induced pulmonary oedema.4 Beside, the changes in Hb during training were not considered, which could account to some extent for the differences in DLCO after training. The first possible explanation is the redistribution of the blood flow to the peripheral tissues after the training through a significant redistribution of fluid shift from the thorax to the peripheral vascular space.13 In our study, we measured DLCO less than 10min after exercise and the decrease in DLCO occurs despite a slight increase in the alveolar volume after training, which conflict with this hypothesis. The second possible explanation is the presence of swimming-induced pulmonary oedema (SIPO) during exercise which has been related to the ultra-structural mechanical stress in the pulmonary capillaries under a condition of high pulmonary artery and capillary pressures14 such as swimming exercise. Currently there is no evidence as to why certain individuals are susceptible to SIPO, although symptoms normally resolve rapidly within 48h7 and do not provoke the development of clinically relevant pulmonary oedema which remains as a rare event.15
In summary, this study shows that swimmers experience subclinical decrease in lung diffusing capacity after training, although elite swimmers have larger lungs and higher diffusing capacity than the general population. Therefore, the swimming-induced decrement in DLCO is a transient phenomenon that does not lead to chronic impairment in pulmonary gas exchange. In fact, we suggest that the highly developed pulmonary function of the elite swimmers could be the result of repeated stress to the alveolar-capillary barrier during training. We also found large inter-individual variability, including some swimmers with a large decrease in lung diffusing capacity after exercise. Therefore, doctors and coaches should pay attention to the individual changes in alveolar-capillary diffusing capacity among elite swimmers exposed to highly demanding training regimes.