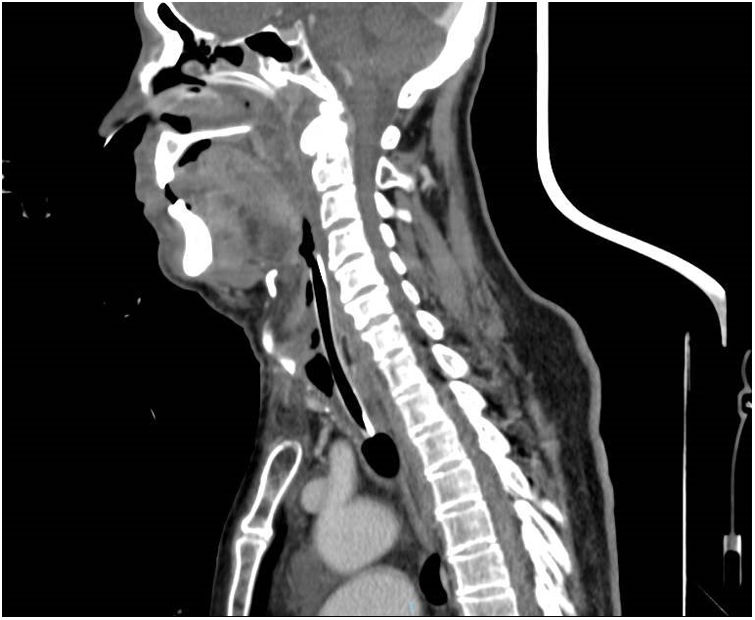
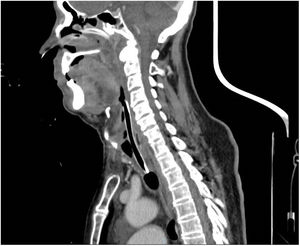
Angioedema is an acute swelling of the deeper layers of the soft tissues or mucosa, caused by increased, localised permeability of the blood vessels.
There are two different conditions of angioedema, namely hereditary and acquired. Hereditary angioedema (HAE) is a rare form of severe angioedema caused by genetic mutations in the complement C1 inhibitor (C1-INH) gene, often leading to a decrease in C1-INH. Clinically, HAE is a disorder characterised by recurrent episodes of severe swelling (angioedema). The worst affected areas of the body are the limbs, face and intestinal tract, whilst airways are affected less frequently.1 Acute attacks can be triggered by infections, emotional stress or trauma, but sometimes no trigger is identified. Acquired angioedema (AAE) typically affects patients over 40 with no family history of angioedema and onsets due to increased consumption (Type 1) or inactivation (Type 2) of C1-INH.
Low serum levels of C1q concentrations differentiate AAE from HAE.2 An 86-year-old man presented at the Emergency Department following the onset of progressive edemas of the face, lips, neck and uvula after administration of the 4th dose of SARS-CoV-2 vaccine COVID-19 Moderna mRNA-1273 72h prior. Previous SARS-CoV-2 vaccinations had been Pfizer-BioNTech. No urticaria was observed and the remainder of the physical examination was unremarkable. In the last eight weeks before the admission, he referred repeated episodes of acute swelling of the upper limbs in the absence of obvious triggers and lasted 3–5 days. At the admission, parenteral corticosteroids, antihistamines and epinephrine aerosol therapy were administrated due to the clinical worsening and rapid development of respiratory failure in the patient, but to no benefit. He underwent a fibro bronchoscopy that showed edemas of the oropharynx and glottis and was subsequently sedated, and urgent orotracheal intubation performed. During the second day of hospitalisation, a surgical tracheostomy was performed owing to the persistence of significative laryngeal soft tissue oedema, evident on CT scans (Fig. 1). Blood tests showed C4 and C1 esterase inhibitor deficiencies (C4 0.02g/l [0.1–0.4g/l]; C1 29% [70–130%]), normal tryptase and histamine. The haematological disease panel showed signs of Monoclonal Gammopathy of Undetermined Significance (MGUS), and the only therapeutic indications were clinical, and laboratory follow up. The patient was treated with tranexamic acid (1500mg/day). In the case described, we believe that AAE was secondary to a haematological disease. It is most likely that the vaccine triggered an acute event in a patient who already had clinical manifestations of AAE.3 Watts MM et al. documented three cases of angioedema after an mRNA Pfizer-BioNTech vaccination that did not require tracheal intubation and improved after medical treatment (steroids, antihistamines). Angioedema onset times varied from 26 to 60h after administration of the vaccine.4 To the best of our knowledge, this is the first report describing the occurrence of AAE after administration of the Moderna vaccination. Physicians examining patients who are to undergo SARS Cov-2 vaccinations should also take into account these less common symptoms and refer patients for further investigation in order to make a correct diagnosis.