COPD is a prevalent disease for which early diagnosis is essential.1 The spirometrically determined ratio of the forced expiratory (FS) volume in 1second (FEV1) over the forced vital capacity (FVC) is the gold standard (GS) test to confirm the presence of airflow limitation. However, FS is frequently underutilized or unavailable in primary care (PC).2–4 Because of easier performance and less variability, several studies have demonstrated that volume at six seconds (FEV6) could be an acceptable alternative to FVC,5,6 and so, the ratio of the FEV1 over the volume measured at 6s (FEV1/FEV6) could be a valid alternative to the ratio FEV1/FVC obtained by FS.7 Zhou et al.,8 in a recent meta-analysis, concluded that micro-spirometers are “user-friendly, patient-friendly, inexpensive, and portable, making them suitable for PC use and providing a feasible pathway for early diagnosis of COPD”; moreover, their use could reduce underdiagnosis of COPD. The European Respiratory Society (ERS) has proposed investigating the role of these devices for early diagnosis.9 The aim of our study was to validate COPD-6® and Piko-6®, the most widely studied micro-spirometers, for COPD screening and to determine the most accurate device for this task.
An observational prospective cross sectional study, calculated to require 569 patients (Epidat 4.2 program) to establish the potential differences between the selected micro-spirometers, recruited a total of 689 patients from the pulmonary outpatient departments at the University Hospitals of Salamanca and Zaragoza in Spain. The inclusion criteria were patients of both sexes, older than 35 years, smokers or ex-smokers with a history of more than 10 pack-years, regardless of whether they had respiratory symptoms. Patients who couldn’t perform valid and repeatable spirometry or had absolute contraindications for the tests were excluded. The study was approved by the Ethics and Clinical Research Committee of the University Hospital of Salamanca.
All spirometry tests were performed by qualified operators according to the ERS/ATS spirometry standards, and were always carried out in the same order (FS, test with COPD-6®, test with Piko-6®). Variables recruited were: anthropometric data, symptoms, FEV1, FVC and FEV1/FVC obtained by FS, used as GS, and FEV1, FEV6 and FEV1/FEV6 obtained by COPD-6® and Piko-6® devices. Personnel conducting the tests ensured that patients rested between tests. Different statistical tests were used on the basis of the variable in question. A significance level of 0.05 was set in all analyses. The statistical tests used to compare the devices were Pearson correlations, Youden Index (YI), Kappa coefficient and ROC curves, and the analysis was performed with software by IBM SPSS 23 version.
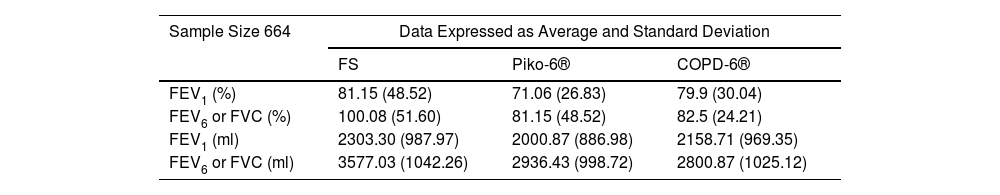
A total of 664 subjects from the total of 689 recruited (491 males, 173 females) met the criteria for inclusion. Average age 61.5±11 years; 338 ex-smokers (51%) and 326 current smokers (49%); average pack-years 41.2±22. Obstruction was defined as FEV1/FVC<70%. FS detected 411 COPD patients (62%), Piko-6® 340 (51%) and COPD-6® 209 (31.5%). Table 1 includes measurements for each variable. Compared to FS, percentages and absolute measurements for FEV1 and FEV6 obtained by hand-held expiratory flow meters were lower. Table 2 shows sensitivity, specificity, PPV, NPV and YI of both devices.
Percentage and Absolute Measurements of FEV1, FVC and FEV6 Obtained by Forced Spirometry (FS), and by the Devices Piko-6® and COPD-6® (Sample Size 664).
| Sample Size 664 | Data Expressed as Average and Standard Deviation | ||
|---|---|---|---|
| FS | Piko-6® | COPD-6® | |
| FEV1 (%) | 81.15 (48.52) | 71.06 (26.83) | 79.9 (30.04) |
| FEV6 or FVC (%) | 100.08 (51.60) | 81.15 (48.52) | 82.5 (24.21) |
| FEV1 (ml) | 2303.30 (987.97) | 2000.87 (886.98) | 2158.71 (969.35) |
| FEV6 or FVC (ml) | 3577.03 (1042.26) | 2936.43 (998.72) | 2800.87 (1025.12) |
ml=milliliters.
Patients Classified as COPD According to GOLD Criteria With the Three Devices: Forced Spirometry (FS), Piko-6® and COPD-6®. Sensitivity, Specificity, PPV, NPV and Youden Index (YI) With Piko-6® and COPD-6®, Reference Test Forced Spirometry (FS).
| Patients Classified as COPD According to Gold Criteria With the Three Devices: Forced Spirometry (fs), Piko-6® and COPD-6® | |||
|---|---|---|---|
| Diagnostic Test FS (Reference Test) | Airway Obstruction (COPD) | No Airway Obstruction (Healthy) | Total |
| 411 | 253 | 664 | |
| Diagnostic Test Piko-6® | Airway Obstruction (COPD) | No Airway Obstruction (Healthy) | Total |
|---|---|---|---|
| Positive | 322 | 18 | 340 |
| Negative | 89 | 235 | 324 |
| Total | 411 | 253 | 664 |
| Value | CI (95%) | ||
| Sensitivity (%) | 78.35 | 74.24–82.45 | |
| Specificity (%) | 92.89 | 89.52–96.25 | |
| Validity index (%) | 83.89 | 81.01–86.76 | |
| PPV (%) | 94.71 | 92.18–97.23 | |
| PNV (%) | 72.53 | 67.52–77.55 | |
| Prevalence (%) | 61.9 | 58.13–65.67 | |
| YI | 0.71 | 0.66–0.76 | |
| Diagnostic Test COPD-6® | Airway Obstruction (COPD) | No Airway Obstruction (Healthy) | Total |
|---|---|---|---|
| Positive | 206 | 3 | 209 |
| Negative | 205 | 250 | 455 |
| Total | 411 | 253 | 664 |
| Value | CI (95%) | ||
| Sensitivity (%) | 50.12 | 45.17–55.08 | |
| Specificity (%) | 98.81 | 97.28–100 | |
| Validity index (%) | 68.67 | 65.07–72.28 | |
| Predicted value + (%) | 98.56 | 96.71–100 | |
| Predicted value − (%) | 54.95 | 50.26–59.63 | |
| Prevalence (%) | 61.9 | 58.13–65.67 | |
| YI | 0.49 | 0.44–0.54 | |
Abbreviations: YI, Youden Index; PPV, positive predictive value; NPV, negative predictive value; FS, forced spirometry; Validity index, diagnostic accuracy: (true positives+true negatives)/total×100.
The Pearson correlation index of FEV1 between FS and Piko-6® and between FS and COPD-6® was 0.94 and 0.97 respectively. Correlations of FEV1/FEV6 Piko-6® and COPD-6® were 0.79 and 0.73. Using FEV1/FVC<70% as a reference, the area under the ROC curve was 0.922 to Piko-6® and 0.913 to COPD-6®. YI of Piko-6® (greater relation between sensitivity and specificity) was higher at cutoff of <73% (YI=0.74). When using COPD-6®, the sensitivity value was lower, so the cutoff point was 80%. The concordance observed between Piko-6® and FS was 83.9%, with a kappa value 0.67±0.028. Moreover, COPD-6® concordance was 68.7% and the kappa value 0.42±0.02.
COPD screening tools are needed to improve disease management. Our study was designed to evaluate the accuracy of Piko-6® and COPD-6® in the diagnosis of airway obstruction and to determine which is more reliable. Jing et al. meta-analyses10 concluded that FEV1/FEV6 has a sensitivity of 89% (IC95%: 83–93%) and specificity of 98% (IC95%: 95–99%) in relation to FEV1/FVC. Several authors11–13 got good results with Piko-6®. In our study, FEV1 and FEV6 acquired by COPD-6® and Piko-6®, both in milliliters (ml) and percentage, were smaller than FEV1 and FVC obtained by FS (p=0.001).
There are two studies with similar objectives and design than ours: Represas et al.14 with COPD-6® and Hidalgo et al.15 with Piko-6®. Our COPD-6® results did not substantially differ from those of Represas. In both, FEV1/FEV6 was larger than FEV1/FVC (p<0.001), which was expected because of FEV6 being lower than FVC. On this basis, the cutoff point for diagnosing obstruction should not be 0.7, since COPD-6® did not detect obstruction in almost half of patients. In our study, we observed smaller differences of FEV1 with respect to FS than Represas, with an average difference of 144ml (IC 95%: 126–162) vs 167ml (IC 95%: 144–190). In contrast, we found a greater difference in FEV6. Similar to Represas, we found a good correlation between COPD-6® and FS, especially for FEV1 measurement.
In Hidalgo's studio and ours FEV1 and FEV6 values with Piko-6® were also smaller than FEV1 and FVC obtained by FS (p<0.001), but there were not significant differences between FEV1/FEV6 and FEV1/FVC. Hidalgo observed a good correlation with FEV1, FEV6 and FEV1/FEV6. Nevertheless, we noticed the best correlation with FEV1 (r=0.94 versus r=0.87) and slightly worse with the ratio (r=0.79 versus 0.94). Correlation could be considered excellent by linear regression lines.
We set out to determine the best FEV1/FEV6 cutoff point in terms of sensitivity and specificity to detect obstruction. However, FEV1/FEV6 acquired by micro-spirometers was greater than FEV1/FVC, and so, a higher cutoff should be considered. To this aim, we used YI. The Piko-6® cutoff point of FEV1/FEV6 was 0.73 while with COPD-6® was 0.8 (considerably lower sensitivity). The cutoff point in the Represas,15 Fritz,11 Hidalgo,14 and Van de Bemt11 studies varied between 0.70 and 0.78. Represas concluded that when using COPD-6®, a cutoff point of 0.7 was not valid for COPD screening, and that a cutoff point of 0.75–0.80 was needed, in accordance with our results.
ROC curves were performed using FEV1/FVC<70% as a reference. For FEV1/FEV6 we noticed AUC 0.91 and 0.92 with COPD-6® and Piko-6®, respectively showing an excellent correlation with FS. Both devices showed an odds ratio higher than 20, the minimum needed to validate a test.
Chen et al.16 stated that micro-spirometry was accurate and had clinical utility. In our research, Piko-6® showed a better concordance than COPD-6® classifying individuals as having COPD or healthy (COPD diagnosis excluded), although a possible limitation of our study is the fact that the tests were always performed in the same order and were not randomized. This study provides real-world evidence to identify best practices when screening for COPD using hand-held devices.
In conclusion, although FEV1 and FEV6 measurements undertaken with hand-held expiratory flow meters were lower than FEV1 and FVC performed with FS, Piko-6® and COPD-6® are useful for COPD screening because correlation with FS is good. A FEV1/FEV6 cutoff point of 0.7 obtained by hand-held expiratory flow meters as COPD screening had false negative results, so, with portable devices, this cut-off point for detecting obstruction must be increased. The usefulness of hand-held expiratory flow meters for COPD screening could help reduce underdiagnoses of COPD and minimize workloads in lung function laboratories. We found Piko-6® to be the device of choice given that it achieves the best correlation with FS. Nevertheless, the exact role of micro-spirometers in the diagnosis process isn’t yet fully established.
Contributing AuthorsMiguel Ángel Hernández Mezquita and Alfonso Pérez Trullen: Study design, case inclusion, analysis of results and supervision of the final manuscript. Idania de Los Santos Ventura, Vanessa Hidalgo Sierra and Enrique Barrueco Otero: case inclusion and initial writing of the manuscript.
Statement of EthicsThis research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and approved by the ethics committee at Salamanca University Hospital on October 2, 2014. Approval code: PI 2014 10 01.
FundingThis work was supported by a research grant from the Spanish Society of Pneumology (SEPAR) – grant code 091/2014.
Conflict of InterestsThe authors have no conflicts of interest to declare.
Data AvailabilityNo restrictions on data availability. Data statement: the data is available at: https://scholar.archive.org/work/ng6ecpnosrftzpkzvkyjhjrhtu.
Thanks to the team at the pulmonary function laboratory at the University Hospital of Salamanca, Jacinto Ramos, head of the unit, and the nurses José Miguel Hernández, Begoña González and Florinda Pérez for his advice.