We report the case of an 11-year-old girl with refractory Crohn's disease treated with ustekinumab, hospitalized for disseminated tuberculosis. This is the first case of disseminated tuberculosis in a child under anti-IL12 - IL23 treatment described in literature.
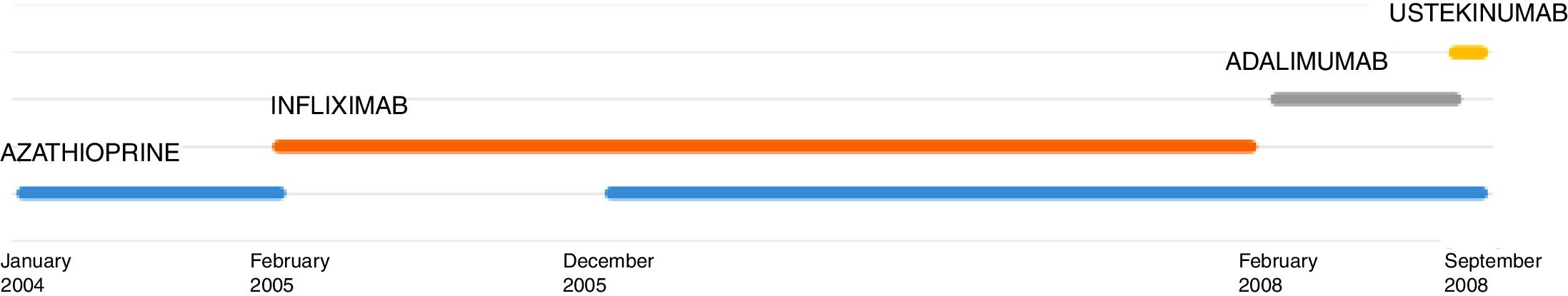
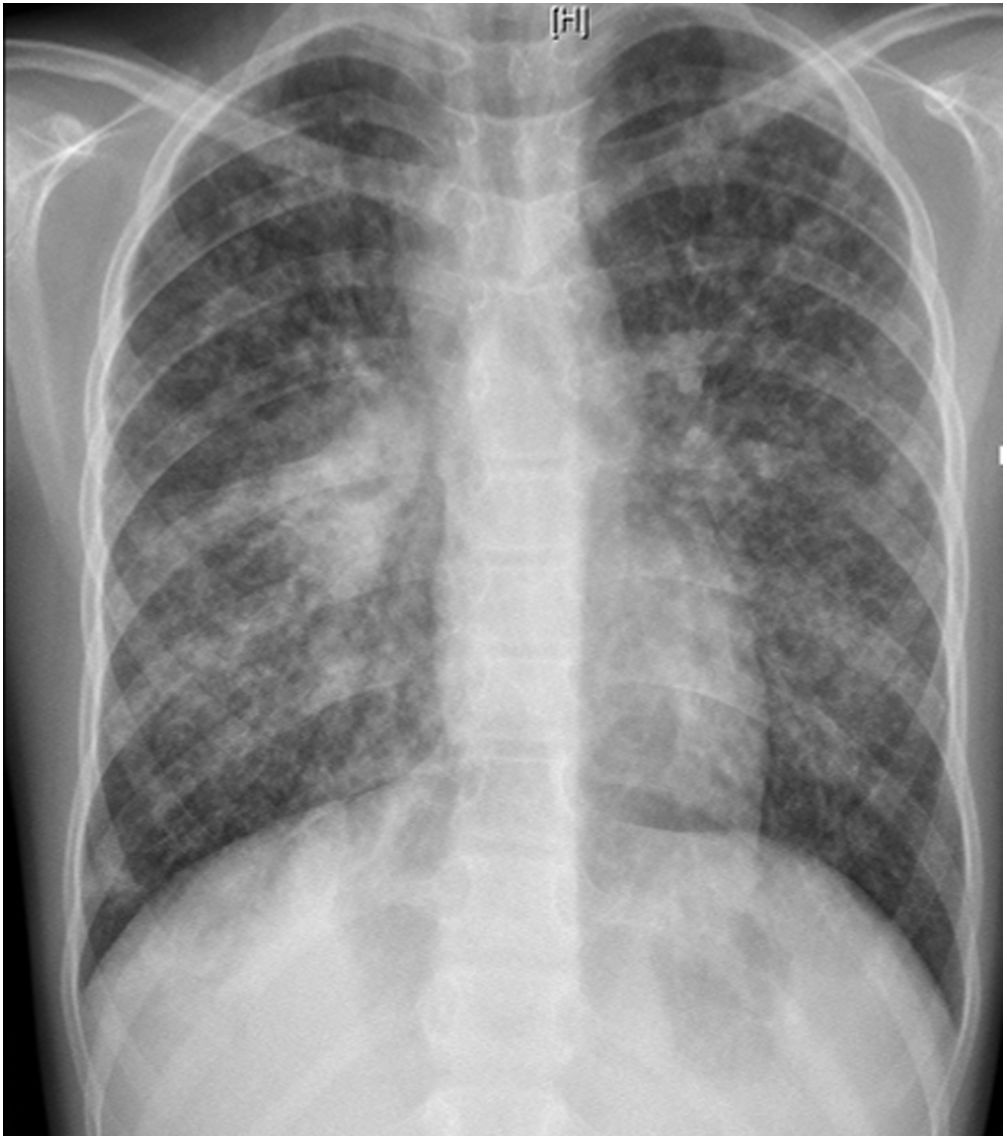
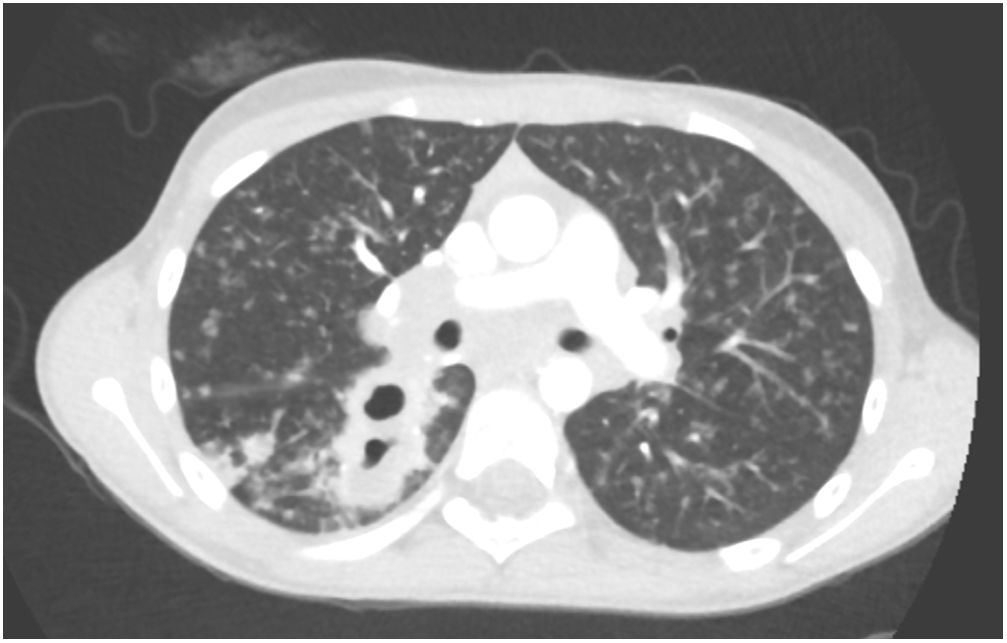
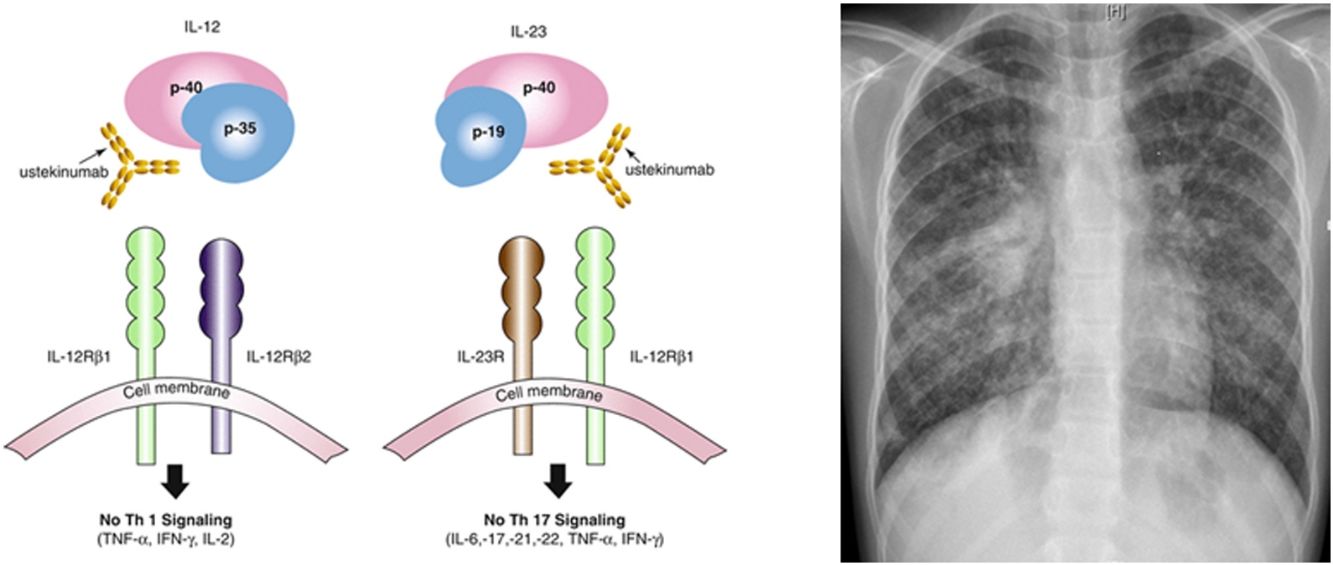
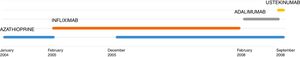
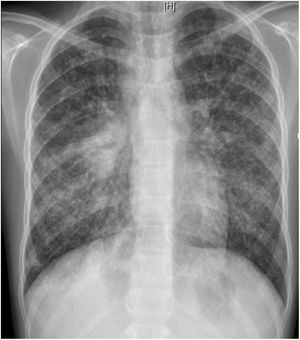
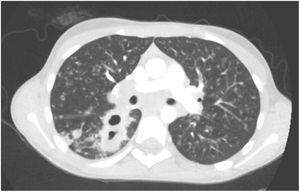
L. was diagnosed with Crohn's disease at the age of 7. She was first treated with oral corticosteroids for 9 months, then azathioprine. Because of persistent symptoms, anti-tumor necrosis factor agents (first infliximab during 3 years, and then adalimumab for 6 months) were added to azathioprine. The child had received BCG vaccination when aged 2 months. The tuberculin skin test was negative, and IGRAs (interferon gamma release assays=QuantiFERON-TB-Gold Cellestis) results were indeterminate before starting anti TNF agents. Abdominal pain, diarrhea, with evening rise fever were persistent despite anti TNF agents. One month after adalimumab was dropped when the child was eleven; ustekinumab treatment was started together with azathioprine, after a collective decision of pediatric and adult gastroenterologists (Fig. 1). No chest X-ray was performed before starting ustekinumab. It was started after 15 days of oral corticosteroids (budesonide 9mg per day). Fifteen days after the first injection of ustekinumab (6mg/kg intravenously), she was referred to our pediatric pulmonology unit for persistent fever, dry cough and weight loss. On clinical examination: oxygen pulse oximetry: 97% (room air); temperature: 39°C; no tachypnea or retraction signs; auscultation showed crackles in the right inferior lobe. Chest X-ray (Fig. 2) showed diffuse alveolo-interstitial opacities, with right parahilar consolidation with central cavitation that is over the helium of 51mm×36mm, and nodules with diffuse distribution of 2–4mm. Blood test showed moderate inflammation (C-reactive protein 80mg/l). Viral and bacterial nasopharyngeal aspirates were negative (Mycoplasma and Chlamydia pneumoniae, multiplex viral PCR). Fiberoptic bronchoscopy showed obstruction of the apical bronchi of the right inferior lobe by endobronchial granuloma. Broncho alveolar lavage showed normal cellularity, with lymphocytosis (=135cells/mm3, with 33% macrophages, 51% lymphocytes, 16% neutrophils). Bacterial culture was negative, as well as respiratory viruses and mycology culture (Pneumocystis jirovecii) on BAL. Acid fast bacilli (AFB) was detected by microscopy using Ziehl Neelsen stain on BAL, sputum smear and post bronchoscopy gastric aspirate. Culture grew for multisensible Mycobacterium tuberculosis. At that time, tuberculin skin test was negative, and IGRA (QuantiFERON-TB-Gold Cellestis) was indeterminate. There were no close contacts with tuberculosis. Thoracic computed tomodensitometry (Fig. 3) was consistent with disseminated tuberculosis. In both lungs there are multiple nodules being the largest in the superior segment of the right lobe where there are bigger than those located in other lobes; multiple adenopathy in the right hilar and interlobar regions; and condensation of 35mm×26mm of the apical segment of the right inferior lobe, with two central excavation of 12mm×5mm and 10mm×7mm.
Because of disseminated tuberculosis, a search for multiorgan involvement was carried out: urinary culture was negative; abdominorenal echography, echocardiography, bone scintigraphy, ophthalmoscopic examination were normal. Cerebral MRI showed punctiform enhancement consistent with a right and left cerebellous granuloma, lumbar puncture was sterile, and neurological examination was normal with no cerebellous syndrom. Tuberculosis treatment was started with 2 months of quadritherapy: isoniazid–rifampicin–ethambutol and pyrazinamide, followed by 10 months of isoniazid and rifampicin (because of cerebral involvement). Azathioprine was temporarily suspended. The young patient was treated with corticosteroids (prednisolone 1mg/kg/day 15 days, with progressive weaning) in prevention of immune reconstitution inflammatory syndrome, and because of bronchial obstruction. Enteral nutrition was added because of undernourishment. On follow up, apyrexia was prolonged, the cough disappeared and the patient gained weight. Chest X-ray after 1 month of treatment was back to normal.
This is the first case described of disseminated TB in a child under ustekinumab for Chron's disease. Disseminated TB in this child was suspected on chest X-ray (and CT scan) and confirmed by the presence of Mycobacterium tuberculosis on sputum smear, BAL, and post FB gastric aspirate.
Miliary or disseminated tuberculosis results from massive lymphohematogenous dissemination of Mycobacterium tuberculosis. Miliary TB represents 1–2% of TB cases. It occurs 2–6 months after initial infection, but can be more precocious in infants. Diagnosing miliary tuberculosis can be a challenge:
- -
Clinical manifestations are nonspecific as in this case. Miliary TB develops less often in children than in infants, especially if BCG vaccination was done.
- -
IGRA or tuberculin skin test can be negative (in 35–74% of miliary cases), as in our case.
- -
Typical chest radiograph findings may not be obvious until late in the disease. Typical findings on chest X-ray are collections of tiny discrete pulmonary opacities that are generally uniform in size and widespread in distribution, each of them measuring 2mm or less in diameter. In 10% of cases, nodules can be wider than 3mm in diameter, as in this case.
- -
Mycobacterial identification is positive in less than 50% of cases with FB.
It is rare in developed countries, and usually happens in infants or immunocompromised children (such as HIV infection)1,2 and in adults after organ transplant, in chronic hemodialysis programs, or in cases of use of immunosuppressive drugs such as anti-TNF. Recently because of novel therapies, cases of tuberculosis have been rising in adult patients under anti-tumor necrosis factor agents, such as infliximab, adalimumab or etanercept.3 In this case, ustekinumab, adalimumab or azathioprine may be responsible of disseminated TB or even the association of these immunosuppressive treatments. A pharmacovigilance study was conducted in this case: imputability score of ustekinumab was 5/6 according to French imputability scoring method.4
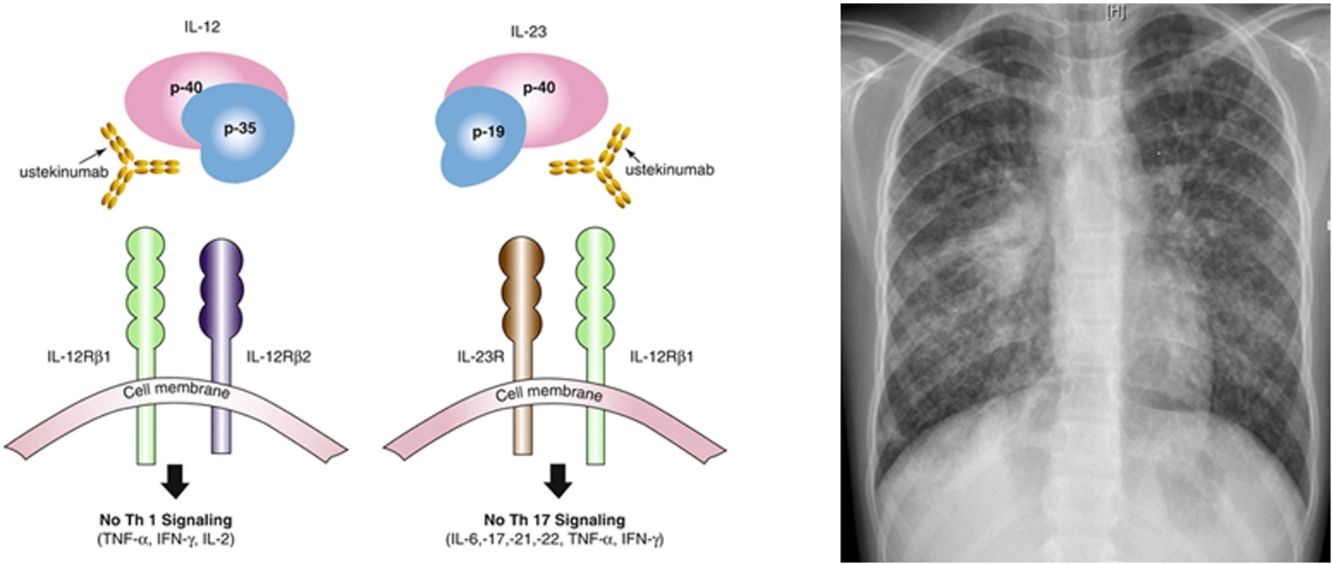
Ustekinumab is a human monoclonal antibody against interleukin IL12 and IL23 p40. It is indicated in moderate to severe Crohn's disease in adults, with incomplete response, loss of response, intolerance of conventional treatment or anti TNF agents, contraindications to these. Its major contraindication is infection, especially TB. Nevertheless, the risk of tuberculosis infection was observed to be lower than in patients who had received anti-TNF agents. Only 11 publications relate drug-induced respiratory disease with ustekinumab: eosinophilic pneumonia, interstitial pneumonia, organizing pneumonia, hypersensitivity pneumonitis, Mycobacterium abscessus infection, Legionella pneumophila pneumonia. Only few cases of TB (latent tuberculosis reactivation, peripheral lymph node recurrence, peritoneal tuberculosis) under ustekinumab in adult patients have been published.6–9 IL12 has a role, so as IL23, in initiation and control of cellular response against TB. Individuals having deficiency in IL12B gene coding for p40 have a mendelian susceptibility to mycobacterial infections. This suggests that IL12/23/INF gamma axis is specifically dedicated in controlling mycobacterial infections, and inhibiting these cytokines can favor such infections. National OMS database “Vigibase” reports 88 cases of TB with ustekinumab being suspected, with no children. Such arguments are in favor of ustekinumab imputability in disseminated TB in this child.
Nevertheless, azathioprine and adalimumab can potentially be implicated in this disseminated TB:
- -
Azathioprine as an immunosuppressive treatment can be responsible of pulmonary infections (viral, fungal of bacterial); but she already had this treatment for many years.
- -
Anti TNF agents: risks of TB, specially under adalimumab are well described. In a prospective study of patients under anti TNF-alpha agents: miliary TB represented 27, 5% of all cases of TB, TB was more frequent in patients under infliximab and adalimumab.3
Before starting ustekinumab, LTBI (latent tuberculosis infection) should be screened. There are few data available on the effect of immunomodulatory/biological therapy on the accuracy of TST and IGRA in BCG vaccinated immunosuppressed patients with inflammatory bowel disease (IBD). IGRA alone was more effective to detect LTBI than TST alone in a Brazilian IBD cohort (n=103) (endemic area for TB, vaccinated population). In addition, IGRA had a ∼60% added value as an add-on sequential test, particularly important in CD patients.5
Although currently there is no gold standard for screening LTBI, especially in the immunosuppressed population, IGRA has become the preferred method in countries with low TB incidence. The combination of TST and IGRA (even using the two commercial IGRAs available, QuantiFERON and T-Spot. TB), can improve the diagnosis sensitivity of LTBI and the initiation of its treatment.10
This is the first case described of disseminated TB in a child under ustekinumab, azathioprine and adalimumab, for Chron's disease.
IGRA and/or tuberculin skin test should be performed before initiating any immunosuppressive treatment in case of Crohn's disease, especially if anti-TNF agents or ustekinumab could be used during follow-up.