The EPISCAN study, published in 2007, was an update of the results of the 1997 IBERPOC study. Changes in demographics and exposure to risk factors demand the periodic update of prevalence and determining factors in COPD. This article is a summary of the protocol and tools used in EPISCAN II.
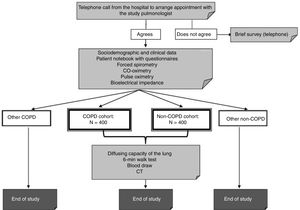
Materials and methodsThe primary objective of EPISCAN II is to estimate the prevalence of COPD among the general population aged 40 years or more in the 17 autonomous communities of Spain. The sample size requires 600 participants (300 men and 300 women) per center, selected by screening 10200 participants in a short visit (questionnaire plus forced post-bronchodilator spirometry). Of these, 800 (400 with COPD and 400 without COPD) will also perform a long visit (including a walking test, blood tests, determination of diffusion, pulse oximetry and bioimpedance, and low radiation CT).
ResultsThe first participant was recruited on 28 February 2017. As of 22 November 2017, a total of 3581 participants had been included, of whom 422 had already performed the long visit. It is estimated that the field work will be completed by December 2018. The new imaging data, biomarkers, and information on new exposures, such as electronic cigarettes and environmental pollution, will help us re-quantify the burden of COPD.
ConclusionsEPISCAN II will provide updated information on prevalence and determinants of COPD in Spain, allowing for the comparison of spirometric results and other factors associated with COPD among the 17 autonomous communities.
En 2007 el estudio EPISCAN actualizó los resultados de IBERPOC en 1997. Debido a los cambios demográficos y en la exposición a factores de riesgo, es importante actualizar los datos de prevalencia y determinantes de la EPOC de forma periódica. El presente artículo resume el protocolo y las herramientas de EPISCAN II.
Material y métodosEl objetivo principal de EPISCAN II es estimar la prevalencia de la EPOC en la población general residente en España de 40 años o más en las 17 comunidades autónomas. El tamaño muestral requiere 600 participantes (300 hombres y 300 mujeres) por centro, seleccionando a 10.200 participantes en visita corta (cuestionario más espirometría forzada posbroncodilatador) y de entre ellos a 800 (400 con EPOC y 400 sin EPOC) que también realizarán una visita larga (prueba de la marcha, sangre, difusión, pulsioximetría, bioimpedancia y TC de baja radiación).
ResultadosEl primer participante se reclutó el 28 de febrero del 2017. A fecha de 22 de noviembre del 2017, contamos con un total de 3.581 participantes incluidos, de los cuales 422 ya han realizado la visita larga. Se estima que el trabajo de campo terminará alrededor de diciembre de 2018. La nueva información de imagen, biomarcadores y nuevas exposiciones, como el cigarrillo electrónico o contaminación ambiental, entre otros, permitirán una nueva cuantificación del problema de la EPOC.
ConclusionesEPISCAN II actualizará la prevalencia y los determinantes de la EPOC en España y permitirá comparar resultados espirométricos y otros aspectos de la EPOC entre las 17 comunidades autónomas.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in Spain. Its economic impact is high, due in part to underdiagnosis that results in most patients going undetected and reaching advanced disease stages while receiving inappropriate treatment. COPD is now understood to be a complex heterogeneous syndrome with both pulmonary and extrapulmonary involvement.1 In clinical practice, the diagnosis of COPD is based on the assessment of exposure to tobacco smoke and other harmful gases, and the presence of respiratory symptoms of chronic airflow limitation, documented with post-bronchodilator spirometry. The severity of airflow limitation, measured by the ratio of post-bronchodilator FEV1 to the predicted value, provides important information for optimizing disease management and establishing severity.1,2
COPD screening should be considered in any individual who presents characteristic disease symptoms and who has been exposed to risk factors, the most important of which is smoking.3
Two studies conducted 10 years apart in Spain, the IBERPOC and EPISCAN, determined a 9.1% prevalence of COPD in the general Spanish population aged 40–69 years,4 and 10.2% in the 40–80-year age range,5 respectively. Other studies, such as PLATINO, found an even higher prevalence (14.3%) in various Latin American countries, also in individuals aged over 40 years.6,7 Despite these figures, COPD is still a disease with high rates of underdiagnosis: estimated rates in Spain were 78% in 1997 and 73% in 2007. The consequence is that diagnosis is made at more advanced disease stages, when the risk of exacerbations and mortality is higher.8
Global mortality estimates indicate that COPD was the fifth cause of death in 1990, and by 2010 it had become the third cause of death,9,10 so an early diagnosis is of vital importance.
We have therefore proposed a new epidemiological study to update our data on the prevalence and determinants of COPD in all autonomous communities in Spain, expanding parameters to include not only spirometry, but also other extra-pulmonary dimensions, with respiratory and non-respiratory questionnaires, a more comprehensive functional evaluation, and in some cases, biological tests such as inflammatory markers, and imaging tests, such as low-dose computed tomography (CT).
The primary objective of EPISCAN II is to estimate the prevalence of COPD in the general population of Spain aged 40 years or more. All secondary objectives are listed in Table 1.
EPISCAN II Study Objectives.
| 1. Primary objective |
| To estimate the prevalence of COPD in the general population of Spain aged 40 years or more. |
| 2. Secondary objectives |
| a. To describe the sociodemographic and clinical characteristics of the study sample |
| b. To describe changes in the prevalence of COPD compared to previous studies |
| c. To describe the geographical variability of COPD prevalence |
| d. To compare the prevalence of COPD among the different age groups and between sexes according to FEV1/FVC post-bronchodilator ratio <0.7 or less than the lower limit of normal |
| e. To evaluate rates of COPD underdiagnosis and overdiagnosis |
| f: To describe comorbidities among the population with and without COPD: Charlson and COTE indices, that associate long-term mortality with patient comorbidities, will be used and complemented with a closed list of other concomitant diseases. |
| g. To describe the distribution of the study population according to GOLD severity classification and by phenotypes and GesEPOC risk stratification. |
| h. To analyze COPD risk factors: level of physical activity, smoking (including degree of smoking using CO-oximetry in a study subsample), workplace exposure, exposure to biomass smoke. |
| i. To evaluate the relationship between COPD diagnosis, symptoms, level of physical activity, and level of smoking |
| j. To evaluate if the treatment received for COPD is in line with the current recommendations, and to describe rates of undertreatment and overtreatment. |
| k. To describe the prevalence of smoking in the population aged 40 years or over in Spain, and by sex and age groups. |
| l. To determine the degree of nicotine dependence among smokers according to the Fagerström test, and the phase of the smoking cessation process of subjects, according to the transtheoretical model of Prochaska and DiClemente. |
| m. To describe the prevalence of respiratory symptoms in the study population |
| n. To evaluate health-related quality of life with the CAT questionnaire (COPD Assessment Test) |
| o. To evaluate possible cognitive impairment among the population aged 60 years or more using the MEC mental state examination (cognitive impairment mini-examination or Mini-mental cognitive examination) |
| p. To evaluate the presence of anxiety and depression among the study population using the Hospital Anxiety and Depression Scale |
| q. To perform a multidimensional assessment of COPD according to BODE and BODEX indices: BMI, dyspnea grade (mMRC), lung function (FEV1), respiratory capacity (walk test), and number of severe exacerbations |
| r. To compare the ratio of lean mass between COPD patients and non-COPD subjects using bioelectrical impedance analysis |
| s. To compare exercise tolerance using the walk test at different levels of COPD severity and in non-COPD subjects |
| t. To compare daily physical activity, evaluated using the Yale Physical Activity Survey questionnaire among COPD patients and non-COPD subjects |
| u. To compare lung attenuation density and airway thickness using CT among the different levels of COPD severity, smokers without airflow limitation, and non-smokers without airflow limitation |
| v. To compare the intensity of dyspnea and other respiratory symptoms, health-related quality of life, exercise tolerance, physical activity, and systemic inflammation among non-COPD subjects and COPD patients, according to their lung diffusing capacity |
| w. To determine the populational prevalence of reduced lung diffusing capacity in subjects without airflow limitation |
| x. In subjects with no evidence of airflow limitation, to compare the impact of a reduced lung diffusing capacity compared to normal diffusing capacity on health-related quality of life, exercise tolerance, systemic inflammation, and lung parenchyma attenuation. |
This is a national, multicenter, cross-sectional, population-based epidemiological study. Study subjects will be selected from the general population of Spain who are resident in the postal code areas nearest to the participating hospitals. The 19 participating hospitals were selected from all autonomous communities (Fig. 1).
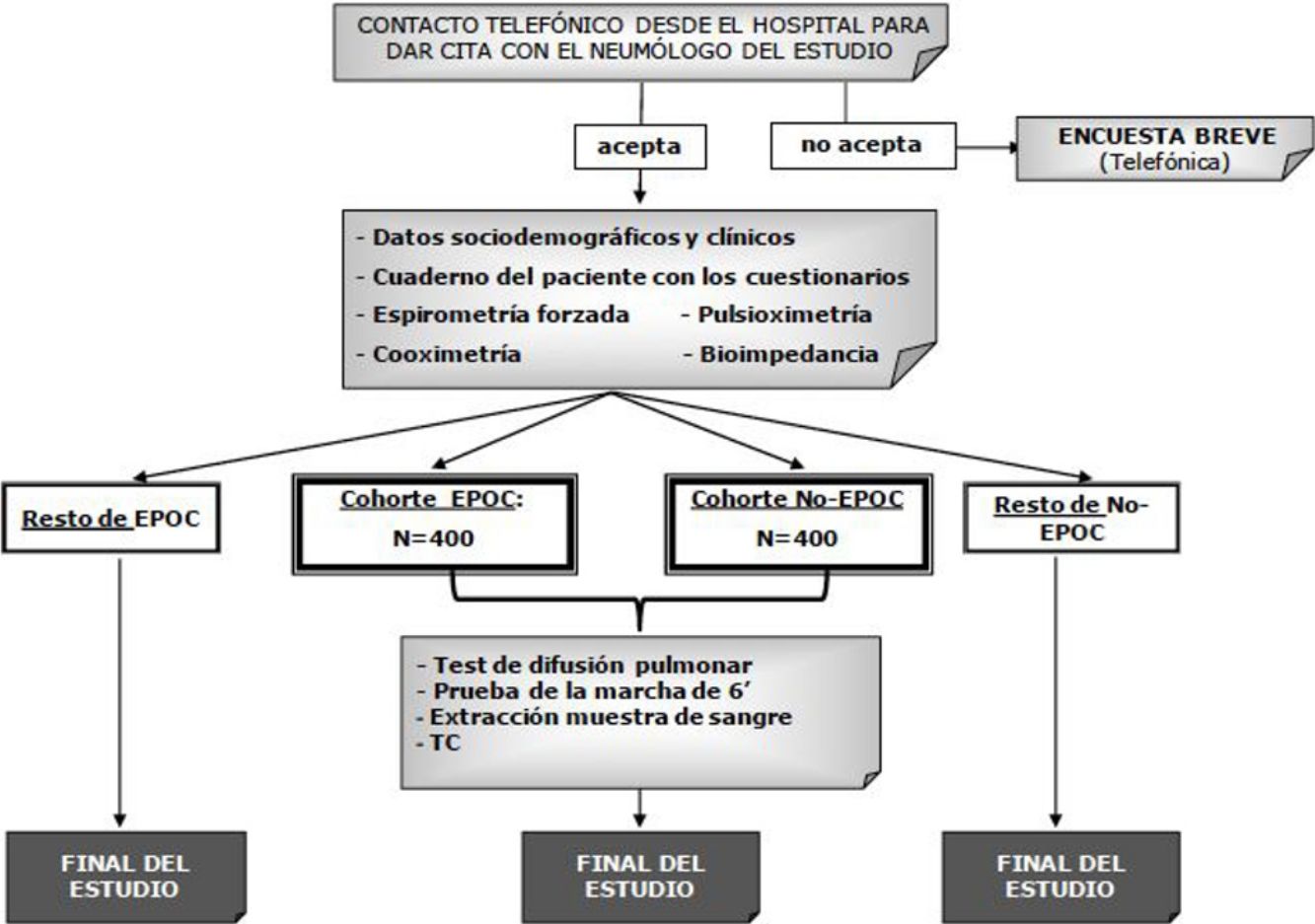
The inclusion criteria are as follows: men or women aged 40 years or more, resident in Spain, with no physical or cognitive difficulties that would prevent them from completing any of the study procedures. A specialized company will contact them by telephone, and if they agree, they will attend the short visit in the hospital center. The content of this short visit is shown in Table 2. Long visits will also be performed in 12 sites, the contents of which are listed in Table 3.
List of Tests and Procedures During the Short Visit.
| Informed consent form |
| Sociodemographic data |
| Baseline and post-bronchodilator spirometry |
| Pulse oximetry |
| CO-oximetry |
| Bioelectrical impedance analysis |
| Participant's notebook |
| CAT |
| HADS scale |
| YPAS questionnaire |
| ECSC questionnaire |
| Questionnaire on workplace exposure |
| Mini-mental (individuals over 60 years of age) |
| Fagerström test (active smokers) |
| Prochaska and DiClemente smoking cessation model (smokers only) |
CAT: COPD Assessment Test; ECSC: European Coal and Steel Community; HADS: Hospital Anxiety and Depression Scale; YPAS: Yale Physical Activity Survey.
The study outline is shown in Fig. 2. The study population will be divided into 2 cohorts, depending on the results of the postbronchodilator spirometry: patients with COPD (FEV1/FVC<0.7) and non-COPD individuals (FEV1/FVC≥0.7).
Selection of ParticipantsStudy sampling will be conducted in 2 stages by an external company (IPSOS) using a pre-selected list of the post codes closest to each hospital. A list of random telephone numbers will be obtained, stratified according to these post codes and quotas for sex and age groups.7 In the second stage, the potential participants will be contacted by telephone and asked some brief questions about their health. They will be asked to allow the hospital to call them to arrange a medical appointment, which will involve the short visit in all cases and the long visit in some sites. If they do not agree to the visit, an attempt will be made during the call to administer a brief 12-question survey on the presence of respiratory symptoms as a STROBE requirement for observational studies.11
The final study population will consist of approximately 10200 participants aged 40 years or more. Between 300 and 600 participants (150–300 men and 150–300 women, depending on whether there are 1 or 2 sites in each autonomous community) should be included in each site. The final study sample will comprise those participants who attend the hospital visit. The study was approved by the ethics committee (EC) of each of the participating centers, with the EC of the Hospital Universitario La Princesa acting as the reference committee. The EPISCAN II protocol is registered at https://clinicaltrials.gov with the No. NCT03028207 and at www.gsk-clinicalstudyregister.com/study/205932.
Study OrganizationA scientific committee composed of 10 pulmonologists and 1 epidemiologist was responsible for study preparation and consultancy. Field work is scheduled to be completed in 2018. In each of the hospitals, a pulmonologist will participate as principal study investigator with a team of co-workers consisting of doctors, nurses and clinical research coordinators. Functional tests and clinical laboratory tests will be conducted by doctors or appropriately trained nursing staff. Imaging tests will be performed at the site itself or in other hospitals, according to the established protocol.
Variables and ProceduresDuring the first telephone call, conducted by an external company, the subject will be informed about confidentiality and data protection, and if they agree to respond, they will be asked questions about their cohabitants, confirmation of the post code for assignment of the nearest hospital, previous diagnoses of respiratory disease (chronic bronchitis, emphysema, COPD, or asthma), smoking habit (smoker and number of cigarettes, never-smoker, former smoker and number of cigarettes) and presence of cough or expectoration. During the second telephone call, conducted by the investigator from the hospital, a survey will be administered that contains questions on the previous diagnosis of respiratory diseases, smoking habit, and the presence of other symptoms associated with COPD. The variables collected during the visit with the healthcare professional will provide a comprehensive profile of both non-COPD individuals who are selected for the study visit, and, in particular, participants in the COPD group.
Sociodemographic Variables (for the Entire Sample)Information will be collected on age, sex, level of education, family conditions, weight and height, and conventional use of tobacco (cigarettes, pipe, cigar) or use of other modes of delivery (electronic cigarette, chewing tobacco, etc.).
Basic Clinical Variables for the Entire SampleBasic information will be collected on each subject's health status, including: concomitant diseases: Charlson index (19 comorbidities)12; COTE index (12 comorbidities)13; previous diagnosis of other respiratory diseases; exacerbations in the last year; dyspnea (modified Medical Research Council scale [mMRC],14 which indicates the subjective dyspnea grade by evaluating the daily tasks that the individual can perform without developing dyspnea); and current treatment for respiratory diseases.
Medical Tests (for the Entire Sample)Spirometry: forced spirometry (Vyntus Spiro, Carefusion, Germany) will be performed as indicated by SEPAR.15
Bronchodilation test: the test will be conducted with the inhalation of 400 μg salbutamol. According to the ATS/ERS guidelines,16 criteria for bronchodilation are an increase in FVC or FEV1>200ml and greater than 12% compared to the baseline value.
Classification using lower limit of normal (LLN): this classification minimizes not only possible false negatives in the spirometric classification of the relatively young population, thereby facilitating the early detection of COPD, but also minimizes false positives in the older population due to the decline in lung volumes, especially FEV1, associated with age, thereby avoiding overdiagnosis.
Baseline pulse oximetry, determined using Pulsox 300i (Konica-Minolta, Japan).
CO-oximetry: the fraction of carbon monoxide in exhaled air will be determined (MicroCO, Carefusion, UK).
Bioelectrical impedance analysis: body composition will be measured on the basis of the electrical properties of the biological tissues (SC240-MA, Tanita, Japan). Parameters will include percentage of fat-free mass, percentage of fatty mass, and percentage of body water.
Sample of 400 COPD and 400 Non-COPD Participants in 12 Preselected SitesSingle-breath CO diffusing capacity (MasterScreen diffusion, Carefusion, Germany): absolute values and percentage of diffusing capacity of the lung for carbon monoxide (DLCO) and alveolar volume will be collected, according to the ATS/ERS recommendations.17
Inflammatory parameters: in addition to routine clinical laboratory tests, 20ml of venous blood will be collected from each participant to determine inflammatory and repair biomarkers and others (Table 4), including alpha-1-antitrypsin deficiency, C-reactive protein, tumor necrosis factor alpha, interleukins 6 and 8, eosinophils, fibrinogen, albumin, nitrites and nitrates. The collection procedure will be standardized and each center must store the samples at −80°C.
Initial List of Biomarkers in Blood to be Explored in EPISCAN II.
| Complete blood count (with hemoglobin, hematocrit, RDW and differential) |
| Basic biochemistry: lipid profile, HbA1c, high sensitivity CRP |
| Alpha-1 antitrypsin |
| Fibrinogen |
| Albumin |
| Procalcitonin |
| Hs-ctnT (high sensitivity troponin), troponin I, NT-proBNP (NTPB natriuretic peptide) |
| IL-1β, IL-1RA, IL-8, IL-6, TNF-α, TNFR I and TNFR-II, IL-13, IL-17A, IL-6R |
| CCL26 (eotaxin-3), CCL17 (TARC), CCL18 (PARC), CCL2 (MCP-1), CCL4 (MPI-1β) |
| IFNγ |
| CXCL10 (PI-10), CXCL11 (I-TAC), CCL3 (MPI-1α), CCL13 (MCP-4) |
| CSF2 (GMCSF), CCL5 (RANTES) |
| Serum Amyloid A1 (SAA) |
| Surfactant protein D (SFTPD) |
| Copeptin |
| Adiponectin |
| Cystatin C |
| Homocysteine CC-16 |
| sRAGE |
| BAFF |
| p16/21 |
| Ku70/80 |
| TERF2 |
| Total klotho (Tklotho) and soluble klotho (Sklotho) |
| Sirtuin-1 |
| WNT5A |
Six-minute walk test: this test will be performed according to the ATS guidelines, with the variation of a single walk to avoid overloading the participants with study procedures. The distance walked will be measured in meters, and the reason for stopping, initial and final heart rate, and oxyhemoglobin (SpO2) saturation will be recorded. The subjective feeling of effort and fatigue in the lower extremities at the end of the test will be evaluated using the modified Borg scale,18 which consists of 12 numerical levels of dyspnea.
Computed tomography (CT): images will be acquired during inhalation, without contrast and with low-dose radiation. The images obtained will undergo semi-automatic post-processing for determination of the percentage of emphysema, areas of extension, airway thickness, aorta diameter compared to pulmonary artery diameter, air trapping, coronary calcifications, bronchiectasis, fibrosis, and other lung parenchyma attenuation and airway wall thickness parameters.19–22
Patient-centered Variables (for the Entire Sample)COPD Assessment Test (CAT)23: health-related quality of life (HRQoL) questionnaire developed to assess the health status of COPD patients, validated in Spanish for the Spanish population. It consists of a single dimension containing 8 items, each of which is answered on a scale of 0 (no limitations) to 5 (severe limitation). The items evaluate the intensity of cough, presence of mucus, chest pressure, dyspnea, limitation in domestic activities, social limitations, and sleep and energy limitations.
Hospital anxiety and depression scale (HADS)24,25: generic anxiety and depression scale designed to assess these 2 constructs in non-psychiatric hospital outpatient clinics, validated for the Spanish population. This is a 14-item questionnaire scored on a 4-point (0–3) Likert scale, divided into 2 subscales – anxiety and depression – each containing 7 items.
Yale Physical Activity Questionnaire (YPAS),26,27 validated for the Spanish population and the elderly population. This questionnaire reflects the amount, frequency, and intensity of physical activity, expressed as metabolic equivalents of task (MET), i.e., MET-h/week, that can be used to estimate the effects of physical activity as a continuous parameter, even at the low levels of activity that might be expected in COPD patients.
European Coal and Steel Community Questionnaire on respiratory symptoms (validated Spanish version)28: this questionnaire consists of 6 sections (cough and expectoration, dyspnea, wheezing and chest tightness, asthma attacks, and treatment [inhalers, suppositories, oral and intravenous medications]), with 26 items in total. This is the reference questionnaire for epidemiological studies on the prevalence of chronic respiratory symptoms in adults.
Questions on exposure in the workplace: these questions will be used to make a semiquantitative estimate of workplace exposure to dusts, gases or fumes, etc., including biomass smoke.
Version adapted and validated in Spanish29 of the Mini-Mental State Questionnaire.30 This is a rapid test for screening and follow-up of dementia. It provides a brief, standardized analysis of the participant's mental status. The items explore 5 cognitive areas: orientation, fixation, concentration and calculation, memory, and language. This questionnaire will be administered only to participants 60 years of age or older.
Fagerström test31,32: administered to smokers. This test evaluates nicotine dependence in smokers. It contains 6 questions with either 2 or 4 possible responses. Although there are no standardized cut-off points, the authors of the Spanish version have published several papers using a cut-off of 6 to indicate high dependence.33
Prochaska's Stages of Change34: this model classifies smokers according to their greater or lesser inclination to make a serious attempt to give up smoking.
Calculation of Sample SizeThe main objective of this study, used to calculate the sample size, is to determine the prevalence of COPD among the population of Spain aged 40 years or more. According to data from the 2011 Census of Population and Dwellings, published by the National Institute of Statistics, this age group comprises 23957645 individuals.
Taking into account the 10.2% prevalence of COPD found in the first EPISCAN study, it is estimated with an accuracy of ±0.6% and a 10% dropout rate, that approximately 10200 eligible individuals will need to be included in the study. According to these calculations, it is estimated that after discounting losses to follow-up, the group of patients with COPD diagnosis will consist of at least 936 individuals and the remaining 8244 subjects will belong to the non-COPD group.
We have decided for logistical reasons to conduct the long visit in 12 centers. Taking into account an alpha error of 5% and a power of 80%, in order to compare percentages between the 2 groups of participants (COPD vs non-COPD), a sample size of 400 subjects per group will be sufficient to detect a relative risk of 1.25 or more for most comparisons.
Statistical AnalysisMean, standard deviation, and minimum and maximum values will be used to describe continuous variables, and, if the distribution of the variable analyzed is non-Gaussian, medians and quartiles will also be presented. Categorical variables will also be described by the number and percentage of patients by response category.
Statistical techniques will be used to ensure compliance with the statistical assumptions before the corresponding parametric tests to compare means and proportions are performed. If the established assumptions are not met, the corresponding non-parametric tests will be used.
Comparative analyses will be performed using the Student's t test for continuous variables, or its non-parametric equivalent, depending on the inherent characteristics of the study variables, and the Chi-squared test for the analysis of categorical variables. ANOVA will be used for the analysis of more than 2 groups. In the post-hoc comparisons, the Bonferroni test based on the Student's t test will be used. A level of significance of 0.05 will be used for all statistical tests performed on the study variables.
All subjects who come to the hospital to perform the study visit and who provide spirometry data, the main study variable, will be considered eligible. Subjects who do not have valid spirometric values or maneuvers will not be included in the calculation of the prevalence of COPD.
The prevalence of COPD and the 95% confidence interval will be calculated from post-bronchodilation spirometric criteria, taking into account that a COPD patient has a FEV1/FVC ratio of less than 0.7. Prevalence will also be presented by sex and age group for the overall sample, and by autonomous community. Patients in the COPD group will then be classified by severity, according to the GesEPOC and GOLD 2017 criteria.
Moreover, for descriptive purposes, the prevalence of COPD will be determined in the general population and by age groups and sex, according to LLN, calculated as the fifth percentile of the normal distribution in a healthy population, thus allowing a comparison of both diagnostic criteria. The reference values of the Global Lung Function Initiative (GLI) will be used.35
A descriptive analysis will be made of the sociodemographic characteristics (age, sex, educational level, marital status, smoking) and the clinical variables (comorbidities, exacerbations, dyspnea, other respiratory symptoms, etc.) of the study population. Results of anthropometric parameters (weight, height, body mass index), mMRC scale, spirometry, pulse oximetry, and additional tests (inflammatory parameters, imaging tests, 6-min walk test, etc.), and the questionnaires completed by participants will be reported.
Characteristics of study participants will be described for the overall study sample, and stratified according to the diagnosis of COPD or non-COPD.
The results obtained in this study will be compared descriptively with those of previous studies such as IBERPOC or EPISCAN, to evaluate differences in the prevalence of COPD and changes over time. Prevalences will reported globally, and by area, sex, and age group.
To determine the risk of developing COPD by sex and age, and other variables that might be associated with this respiratory disease, a multivariate analysis will be performed using logistic regression, taking the presence of COPD as a dependent variable, and age, sex, educational level, smoking, comorbidities, and others as independent variables, adjusted for hospital of origin.
To evaluate rates of underdiagnosis, a 2×2 table of prior knowledge of the presence or absence of COPD and COPD diagnosis in the study will be prepared. The concordance between both variables will be analyzed using the Kappa index and other methods.
Subjects will undergo a comprehensive assessment of possible comorbidities and the presence and prevalence of specific respiratory diseases, such as chronic bronchitis, emphysema, previous COPD, and asthma.
HRQoL will be determined according to the CAT questionnaire, stratified by COPD diagnosis. HRQoL indices will be compared among the 2 study groups.
The treatment received by the COPD population will be evaluated to determine if it is in line with the current recommendations, and the rate of undertreatment will be described.
Secondary study objectives will include a description of the prevalence of smoking and the use of electronic cigarettes in the Spanish population aged 40 years or more, overall and by sex, and the association between the COPD diagnosis, level of physical activity, and smoking, by study cohorts.
Fagerström test scores obtained in the subpopulation of smokers and their current “stage of change” according to the Prochaska assessment will be reported.
The findings of CT imaging tests performed in a study subsample will be described.
DiscussionSince 2007, COPD prevalence data in the general population in Spain have been scant. Indeed, since the EPISCAN study was published in 2009, no other publications have appeared on the prevalence of COPD in a large sample of patients in Spain, with the exception of a study carried out in the Canary Islands (Gran Canaria and Tenerife) that reported a COPD prevalence of 7.3% (95% CI: 5.5–9.5), with a level of underdiagnosis of 71.6%, and undertreatment of 63.5%.36 However, other case-finding studies have confirmed COPD underdiagnosis in a series of selected populations.37,38 For these reasons, the EPISCAN II study was designed with the main objective of updating information from the first EPISCAN study to the situation in 2017.
We aim to take advantage of the large sample of individuals with COPD expected in this study to explore some new secondary objectives not included in the first study, including a description of the level of physical activity according to COPD severity, and a description and analysis of other aspects of this disease, such as anxiety or depression, symptoms that are commonly associated with COPD but rarely diagnosed,39 and for which varying prevalence rates have been reported. This study will also assess the impact of COPD on symptoms and HRQoL. This was already found to be significant in 2007, due to the high level of disability caused by symptoms and reduced physical capacity.40 This time we will also use the CAT questionnaire. Loss of HRQoL is an important marker in these patients because it reflects the impact of the disease on their lives and contributes additional information to the clinical evaluation of respiratory disease that may be of value in the management and evaluation of COPD patients. HRQoL dimensions associated with sleep, mobility and physical function are the most severely affected in the majority of studies, and in large part these limitations are due to dyspnea41; moreover, physical function and social activities are aspects that tend to get worse as severity, measured by the GOLD classification, increases.42
Spirometry will be carried out according to the ATS/ERS recommendations,16 with daily calibration of the equipment with a certified 3 l syringe, and use of a disposable anti-bacterial filter. Maneuvers will be considered acceptable when the back extrapolated volume is less than 0.15l or 5% of FVC, expiration lasts at least 6s, and less than 0.025l is exhaled in the last second.15,16 Both acceptability and repeatability of the pre- and post-bronchodilator spirometries will be classified according to their level of quality.2 For this study, only tests classified as having quality A, B and C will be accepted (A: 3 acceptable maneuvers with a difference between the 2 best FVC and FEV1 of less than or equal to 0.15l; B: 3 acceptable maneuvers with a difference between the 2 best FVC and FEV1 of less than or equal to 0.2 l; or C: 2 acceptable maneuvers with a difference between the 2 best FVC and FEV1 of less than or equal to 0.2l).15 GLI reference values35 will be used to interpret the maneuver, and in line with the ATS/ERS guidelines, bronchodilator tests will be considered positive when FEV1 or FVC increases by more than 200ml and more than 12% compared to the pre-bronchodilator value.16
Quality control of measurements is essential for obtaining valid, reproducible results in any study, including observational prevalence studies, such as EPISCAN II. Certain elements introduce variability: the patient, the technician (and their training), and the quality and maintenance of the test equipment. All these factors must be controlled in accordance with the STROBE guidelines,11 as follows: (1) pre-study conditions must be respected (e.g., smoking and the use of bronchodilator medication must be avoided before performing spirometry), in order to reduce patient-dependent sources of variability; (2) the test must be performed in accordance with the protocol and international standards (e.g., impedance, etc.); (3) results must be interpreted using the appropriate reference equations (equations described below); (4) equipment must be calibrated (volume and analyzers); and (5) quality control of analyzers is required (biological controls, simulator).
The limitations of the EPISCAN II study are typical of a cross-sectional study designed to estimate the prevalence of COPD and describe it at a populational level, so it will be impossible to analyze patient progress in terms of COPD or other variables such as HRQoL. Another limitation of a cross-sectional design is that, while we can estimate prevalence as planned in the study and determine associations with certain factors, we cannot associate causality with other variables. Despite these limitations, we decided to use this design to facilitate the participation of individuals from the general population, instead of the hospital population, to enable them to be compared with the IBERPOC and EPISCAN series and others.43
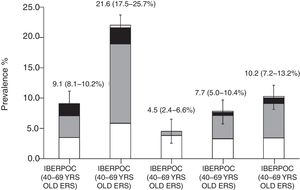
As mentioned above, 2 previous studies, IBERPOC6 and EPISCAN,7 were performed in Spain, 10 years apart. Although the difference in the prevalence of COPD in these 2 studies seems to be small (9.1% and 10.2%, respectively), a formal comparison reveals an objective decrease in COPD if it is defined using the same spirometric criteria44 (Fig. 3). A generalized decrease in death due to COPD has also been observed in Spain, Europe,45 and worldwide.11
Changes in the prevalence of COPD between IBERPOC (1997) and EPISCAN (2007), according to different spirometric criteria.
Reproduced with permission from Soriano et al.44
Changes in the prevalence of COPD in our setting are a reflection of cumulative exposure to smoking. During the last decade, health policies have changed with the introduction of the Anti-Smoking Act 28/2005 of 2006 and its subsequent modification by Act 42/2010, in 2011.46 It is very likely that these primary prevention healthcare measures have contributed significantly to the decrease in COPD prevalence, since smoking in our environment is still the main cause of COPD. Another significant finding highlighted by both studies was underdiagnosis, a practically universal phenomenon in COPD.45 In this new study, we hope to find a reduction in the rates of underdiagnosis, and an absence of overdiagnosis.47 If COPD is diagnosed in the early stages, therapeutic and preventive measures can be introduced, preventing the disease from progressing to more advanced stages that involve greater disability and increased healthcare spending.1,2
Moreover, since the first EPISCAN study, new smokeless forms of tobacco consumption have appeared, such as the electronic cigarette, and it will be interesting to assess the impact of this device on smoking among the population and on the development of lung disease.
As in a previous analysis between IBERPOC and the first EPISCAN study,44 it will be impossible to directly compare changes in results from the new EPISCAN II study with the IBERPOC and EPISCAN results, and a process of translation and interpretation of all the data will be required, since the age thresholds, some protocol changes in the spirometry maneuvers and the bronchodilator test, and reference equations, differ among the 3 studies.
Respiratory medicine experts and other COPD specialists have always dreamt of identifying a biological marker. Although no useful blood biomarker has been found to date, perhaps other new parameters or imaging biomarkers could be explored, for example, airway changes or the early presence of emphysema with normal lung function results.
COPD is an important cause of death, but so is lung cancer, and this too is still diagnosed at advanced stages, when therapeutic options are no longer possible. Thus, since both diseases have the same risk factors, and smokers with COPD have a higher risk of lung cancer than smokers without COPD, using imaging techniques in this study may help us detect early stage lung cancers.
In conclusion, EPISCAN II will update the prevalence, distribution, and determining factors of COPD in Spain, and will allow us to compare for the first time spirometric results and other factors associated with COPD among the 17 autonomous communities of Spain.
Highlights- 1.
Data on the prevalence and determinants of COPD must be regularly updated.
- 2.
EPISCAN II will estimate the prevalence of COPD in the general population of Spain aged 40 years or more in the 17 autonomous communities of Spain.
- 3.
The estimated sample size of 10200 participants will allow a thorough exploration of the problem and its determinants.
EPISCAN II is funded by GSK (GSK ID: 205932; NCT03028207).
Conflict of InterestsImmaculada Alfageme has participated in conferences, advisory committees and consultancies sponsored by AstraZeneca, Boehringer Ingelheim, Esteve, GEBRO, Grifols, GSK, Novartis, Pfizer, Roche, and Teva during the period 2014–2018.
Pilar de Lucas has had no conflict of interest in the last 3 years.
Julio Ancochea has received honoraria from Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Linde Healthcare, Menarini, Mundipharma, Novartis, Roche, Rovi, Sandoz, and Teva during the period 2014–2018 for speaking at conferences, moderatorships, scientific consultancy services and participating in clinical trials.
Marc Miravitlles has received speaker honoraria from Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, CSL Behring, Grifols, Novartis and Zambon; consultancy fees from Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratories Esteve, Mereo Biopharma, Verona Pharma, pH Pharma, Novartis, Grifols, and Teva; and research grants from GlaxoSmithKline and Grifols.
Juan José Soler-Cataluña has received honoraria from Air Liquide, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Menarini, Mundipharma, Novartis, Rovi, Sandoz, Teva, and Zambon for speaking engagements, scientific consultancy, and participation in clinical studies.
Francisco García-Río has received honoraria from (in alphabetical order) AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GEBRO, GlaxoSmithKline, Linde, Menarini, Novartis, Rovi, and Teva from 2014 to date for speaking engagements, scientific consultancy, participation in clinical trials, and preparation of manuscripts.
Ciro Casanova has received honoraria from (in alphabetical order) AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Gebro, GlaxoSmithKline, Menarini, Novartis, Rovi, and Teva in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and preparation of manuscripts.
José Miguel Rodríguez González-Moro has received honoraria from (in alphabetical order) AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Menarini, Novartis, Orion, Rovi, Teva, and Zambón in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and preparation of manuscripts.
Borja G. Cosío has received honoraria from (in alphabetical order) AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Menarini, Novartis, Rovi, Teva, and Zambón in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and preparation of manuscripts.
Guadalupe Sánchez is an employee of GSK.
Joan B. Soriano has received research grants from 2014 to date from Linde through the intermediary of the Hospital Universitario de La Princesa, and has participated in conferences, advisory committees, and consultancy boards sponsored by Almirall, AstraZeneca, Boehringer Ingelheim, CHEST, Chiesi, ERS, Esteve, GEBRO, Grifols, GSK, Linde, Lipopharma, Mundipharma, Novartis, Pfizer, RiRL, Rovi, Sandoz, SEPAR, and Takeda during the period 2014–2018.
We are grateful for the voluntary participation of all the individuals randomly selected for participation in the EPISCAN II study. We explicitly thank Mónica Sarmiento of IQVIA, Maria Victoria Pardo, Miguel Pascual, and David Banas of GSK for their contribution. EPISCAN II is funded by GSK.
Scientific committee: Inmaculada Alfageme, Pilar de Lucas, Julio Ancochea, Marc Miravitlles, Juan José Soler-Cataluña, Francisco García Río, Ciro Casanova, José Miguel Rodríguez González-Moro, Borja Cosio, Guadalupe Sánchez and Joan B Soriano
Principal investigators, co-workers, and participating centers
| AC | Hospital | Study team |
|---|---|---|
| Madrid | H. La Princesa | Julio Ancochea Bermudez (PI)/Elena García Castillo/Claudia Valenzuela |
| Castile and Leon | H. U. de Burgos | Ana Pueyo Bastida (PI)/Lourdes Lázaro Asegurado/Luis Rodríguez Pascual/M. José Mora |
| Aragon | H. Gral. San Jorge | Luis Borderias Clau (PI)/Lourdes Arizón Mendoza/Sandra García |
| Extremadura | H. San Pedro de Alcántara | Juan Antonio Riesco Miranda (PI)/Julián Grande Gutiérrez/Jesús Agustín Manzano/Manuel Agustín Sojo González |
| Castile and Leon | H. Clínico U. de Salamanca | Miguel Barrueco Ferrero (PI)/Milagros Rosales |
| Galicia | H. Álvaro Cunqueiro | José Alberto Fernández Villar (PI)/Cristina Represas/Ana Priegue/Isabel Portela Ferreño/Cecilia Mouronte Roibás/Sara Fernández García |
| Balearics | H. Son Espases | Borja G Cosío (PI)/Rocío Cordova Díaz/Nuria Toledo Pons/Margalida Llabrés |
| Aragon | H. U. Miguel Servet | José María Marín Trigo (PI)/Marta Forner/Begoña Gallego/Pablo Cubero/Elisabet Vera |
| Community of Valenciana | H. Arnau de Vilanova (Valencia) | Juan José Soler Cataluña (PI)/M. Begoña Picurelli Albero/Noelia González García |
| Andalusia | H. Virgen de la Macarena | Agustín Valido Morales (PI)/Carolina Panadero/Cristina Benito Bernáldez/Laura Martín -Bejarano and Maria Velarde |
| Murcia | H. Gral. U. Santa Lucía (Cartagena) | Antonio Santa Cruz Siminiani (PI)/Carlos Castillo Quintanilla/Rocío Ibáñez Meléndez/José Javier Martínez Garcerán/Desirée Lozano Vicente/Pedro García Torres/Maria del Mar Valdivia |
| Navarre | Clínica Universidad de Navarra | Juan Pablo de Torres Tajes (PI)/Montserrat Cizur Girones/Carmen Labiano Turrillas |
| La Rioja | H. de San Pedro (Logroño) | Carlos Ruiz Martínez (PI)/Elena Hernando/Elvira Alfaro/José Manuel García/Jorge Lázaro |
| Basque Country | H. Santiago Apóstol (H. Txagorritxu) | David Bravo (PI)/Laura Hidalgo/Silvia Francisco Terreros/Iñaki Zorrilla/Ainara Alonso Colmenero |
| Asturias | H. Central de Asturias | Cristina Martínez González (PI)/Susana Margon/Rosirys Guzman Taveras/Ramón Fernández/Alicia Álvarez |
| Cantabria | H. de Valdecilla (Servicio de Neumología en el H. Santa Cruz de Liencres) | José Ramón Agüero Balbín (PI)/Juan Agüero Calvo |
| Catalonia | H. U. Vall d’Hebron | Jaume Joan Ferrer Sancho (PI)/Esther Rodríguez González/Eduardo Loeb |
| Castile-La Mancha | H. U. de Guadalajara | José Luis Izquierdo Alonso (PI)/M. Antonia Rodríguez García |
| Canary Islands | H. U. de Tenerife | Juan Abreu González (PI)/Candelaria Martín García/Rebeca Muñoz/Haydée Martín García |
AC: autonomous community; H., Hospital; H. Gral., General Hospital; H.U., University Hospital; PI, principal investigator.
Please cite this article as: Alfageme I, de Lucas P, Ancochea J, Miravitlles M, Soler-Cataluña JJ, García-Río F, et al. Nuevo estudio sobre la prevalencia de la EPOC en España: resumen del protocolo EPISCAN II, 10 años después de EPISCAN. Arch Bronconeumol. 2019;55:38–47.