Birt-Hogg-Dubé syndrome (BHDS) is a rare clinico-pathological entity, named after the 3 Canadian doctors who first described it in 1977.1 Prevalence is estimated at 1/200000 births. The underlying cause is a mutation located on chromosome 17p11.2 of the FCLN gene that encodes folliculin.2 It is characterized clinically by the presence of skin lesions, lung cysts that may be associated with recurrent pneumothorax, and kidney tumors.3 We report the case of a patient who was diagnosed with BHDS after presenting multiple recurrent episodes of pneumothorax.
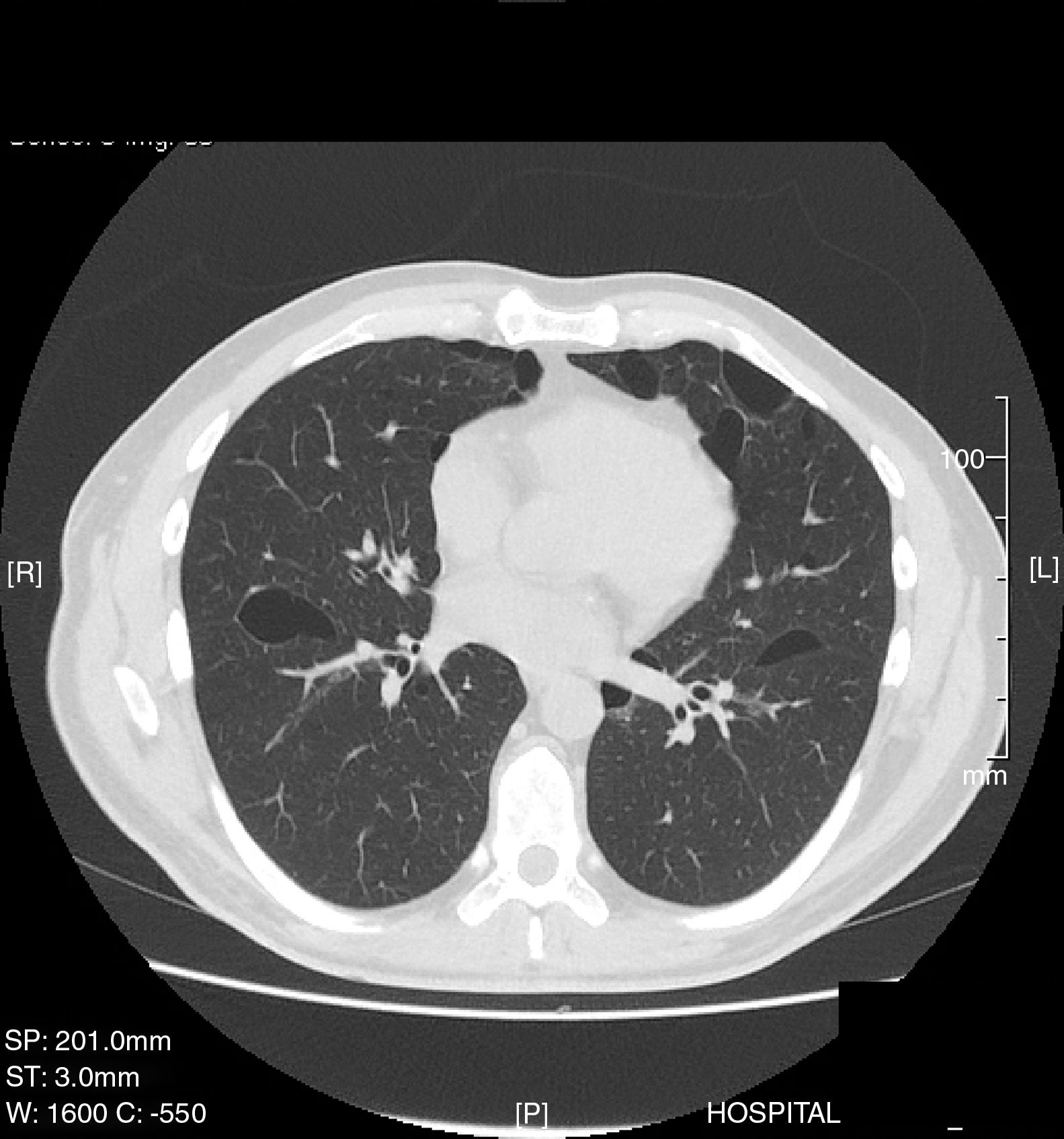
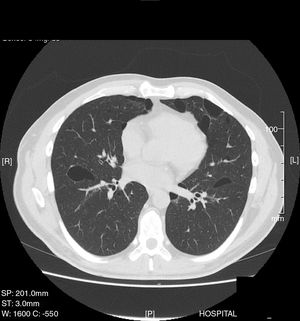
This was a 35-year-old man, non-smoker, with a clinical history of arterial hypertension and bronchial asthma, who was admitted to the emergency department with a 1-week history of right scapular pain. Chest X-ray revealed complete right pneumothorax with no other lung abnormalities, so a chest tube was placed and progress was favorable. Thirty days later, he presented a new episode of complete right pneumothorax, so a video-assisted thoracoscopy was performed in the right side, revealing the presence of small apical bullae. These were resected, and mechanical pleural abrasion of the upper third of the hemithorax was performed. The patient progressed favorably and was discharged from hospital three days after the intervention. The histological study results were consistent with emphysematous bullae. Four years later, the patient presented in the emergency department again with left pleuritic pain, and a complete left pneumothorax was diagnosed. Chest CT was performed, revealing pneumothorax and multiple, large bilateral cystic cavities, predominantly in the lung bases (Fig. 1). Given the patient's history of previously treated contralateral pneumothorax, left video-assisted thoracoscopy was performed, with resection of the apex and mechanical pleural abrasion of the upper third of the hemithorax. Histological study of the resected pulmonary apex found emphysematous bullae, with no other changes. The patient progressed favorably and was discharged from hospital three days later. Two years later he was readmitted with recurrence of complete right pneumothorax, so video-assisted thoracoscopy was performed again, and chemical pleurodesis was applied with 8g of talc. Given the recurrent, bilateral nature of the pneumothorax episodes and the CT image of bilateral cysts, a detailed clinical exploration was performed. Notably, discrete papular skin lesions of microcystic appearance were found on the patient's forehead. These were biopsied and determined on histology to be fibrofolliculomas. The recurrent bilateral pneumothoraxes, bilateral pulmonary cystic cavities, and fibrofolliculomas of the skin suggested the possibility of BHDS, so a study of peripheral blood for the FLCN gene was performed, as mutations in this gene are associated with the appearance of this syndrome. A change in exon 11 of the FLCN gene in position 1285, consistent with a cytosine deletion, was detected. This alteration involves the introduction of a premature stop codon, resulting in a truncated protein. Because this syndrome is associated with kidney tumors, an abdominal CT was performed, which was normal. The patient has had no recurrence of pneumothorax to date. A more comprehensive histological analysis of the lung tissue obtained during the first two surgical interventions was requested. The new report confirmed that the pulmonary bullae in both the right and apices were surrounded by normal alveolar walls, protruding into the interlobular septa. These histologic features have been described (along with the presence of intracystic septa and profusion of venules in the cystic space) as characteristic of BHDS.4
BHDS is a rare autosomal dominant genodermatosis characterized mainly by cutaneous fibrofolliculomas and/or trichodiscomas, pulmonary cysts, spontaneous pneumothorax, and kidney tumors. The gene involved in this syndrome, FLCN, encodes folliculin, which is expressed mainly in the skin, kidneys, and lung.5 The main criteria for the diagnosis of BHDS are FLCN mutations on the genetic study, and the presence of skin lesions (fibrofolliculomas or trichodiscomas).6 The most common extracutaneous manifestations are respiratory: up to 80% of BHDS patients have pulmonary cysts which can remain asymptomatic for years.7 The number and size of the lesions varies from one patient to another, ranging from small cysts to bullae measuring several centimeters, located mainly in the lung bases and in the subpleural region. Larger cyst size and volume have been associated with a greater risk of developing pneumothorax. Approximately 20%–30% of patients with pulmonary cysts have a history of around two episodes of pneumothorax. Moreover, most patients with a history of pneumothorax have been reported to have multiple pulmonary cysts. The right lung is more often affected, although both lungs may be involved in up to 23% of cases.8 A recent study found that 5%–10% of spontaneous primary pneumothoraxes may be related with BHDS.9 The pathophysiology of the pulmonary cysts is unknown. The most current theory is the “stretch hypothesis”10 which suggests that the cysts may originate from cell–cell adhesion defects generated by the mutation. Over time, repeated pulmonary expansion “stretches” the alveolar spaces, particularly in the regions of the lung with larger changes in alveolar volume. Chest CT is the examination of choice for the diagnosis of lung involvement.11,12
The prevalence of renal tumors in these patients varies from 6.5% to 34%, depending on the study,7 with a predilection for men and the 20–55 age group.13 Kidney lesions are usually bilateral and multifocal, with some specific histologic types, five of which are: hybrid forms of oncocytoma and chromophobe renal cell carcinoma (50%), and pure forms of chromophobe carcinoma (34%), oncocytoma (5%), clear cell (3%), or papillary (2%).7,14
Although some authors have associated BHDS with colon cancer, no specific indication for colonoscopy has been described in these patients, and recommendations are the same as for the general population.15
In conclusion, a patient who presents with multiple recurrent pneumothoraxes who shows bilateral pulmonary cysts on CT should undergo a dermatological examination to detect accessible skin lesions for biopsy and genetic study. This diagnosis justifies a study of the abdomen, and patients should be monitored for the early detection and treatment of kidney tumors.
Please cite this article as: Fibla Alfara JJ, Molins López-Rodó L, Hernández Ferrández J, Guirao Montes A. Neumotórax espontáneos de repetición como presentación del síndrome de Birt-Hogg-Dubé. Arch Bronconeumol. 2018;54:396–397.