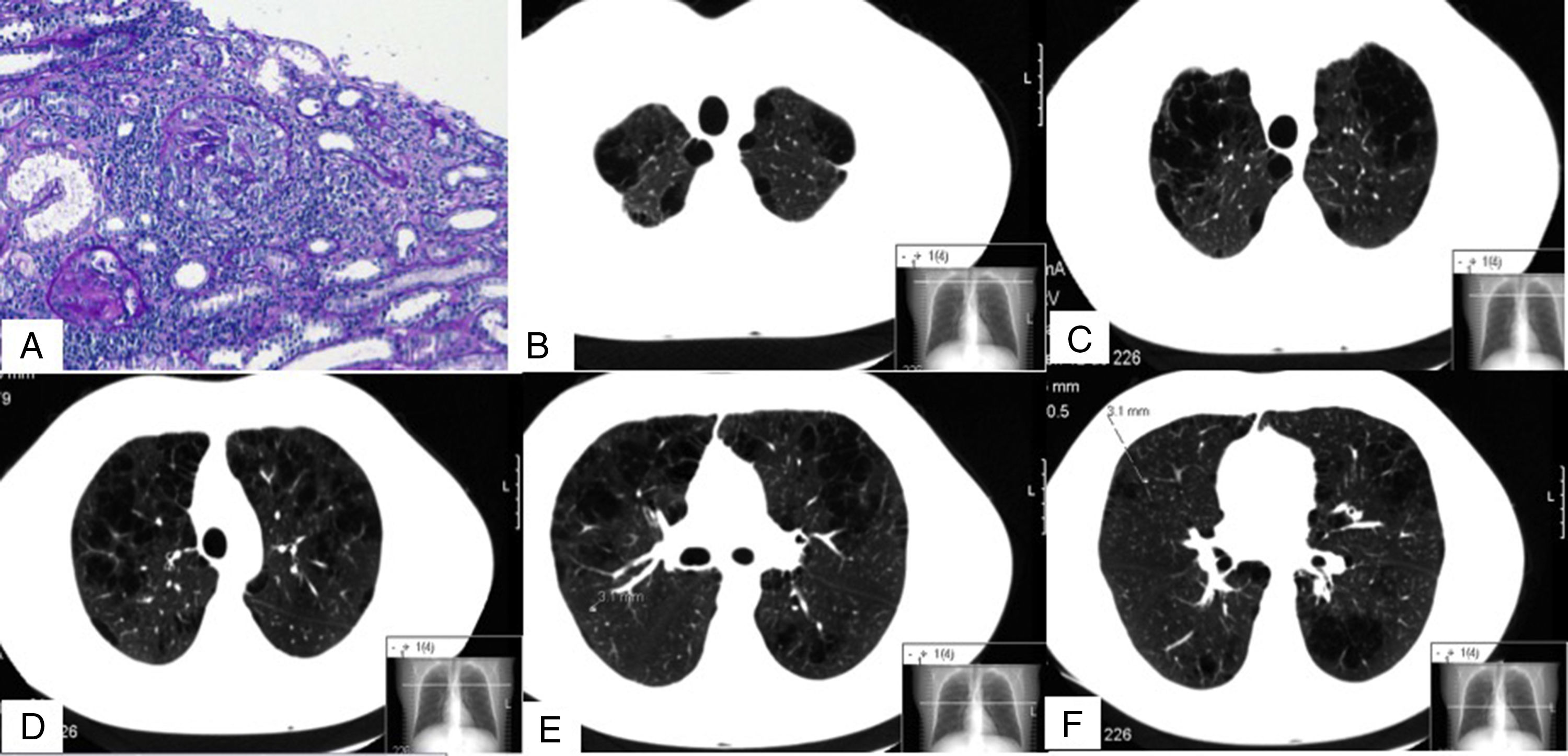
Pulmonary involvement is common in ANCA-associated vasculitis (AAV), but rarely manifests as pulmonary emphysema (13% of cases).1,2
We report the case of a 32-year-old man, smoker of 13 pack-years, with no exposure to other toxic substances, no family history, and no significant clinical history, who was diagnosed with anti-proteinase 3 (PR3) c-ANCA vasculitis and severe pulmonary emphysema. At the time of diagnosis, the patient had constitutional symptoms, arthralgia, digital ischemia, and kidney diseases in the form of non-nephrotic proteinuria, and microhematuria with normal glomerular filtration. Clinical laboratory tests revealed hemoglobin 12.2g/dl and elevated ESR and C-reactive protein. The immunological study was positive for c-ANCA, with an anti-PR3 titer of 79U/ml (normal value <2U/ml) and anti-MPO 0U/ml. Other studies, which included anti-glomerular basement membrane antibodies, ANA, complement, immunoglobulins, cryoglobulins, antiphospholipid antibodies, proteinogram, and hepatitis B, C, and HIV serologies, were normal or negative. Mantoux and Quantiferon® were negative. Kidney biopsy showed pauci-immune extracapillary proliferative glomerulonephritis with crescent formation in 46% of the glomeruli (Fig. 1A). ENT computed tomography revealed no significant changes; chest CT showed 3 nodules <5mm in the right lung, and severe bilateral diffuse mixed centrilobular emphysema with areas of paraseptal involvement and subpleural bullae, mainly in the upper lobes (Fig. 1B–F). No siderophages were found in sputum. Of note on lung function tests were: DLCO: 68%; KCO: 66%; FEF 25%–75%: 58%; FEV1: 80%; and FEV1/FVC: 69%. He was treated with glucocorticoids at a starting dose of 1mg/kg/day p.o. in a tapering schedule, and intravenous cyclophosphamide according to the CYCLOPS scheme.3 The patient stopped smoking and began treatment with bronchodilators. Alpha-1 antitrypsin levels were determined twice, and were normal on both occasions (140 and 145mg/dl, respectively). PI*S and Pi*Z alleles of the AAT gene were also determined qualitatively using PCR-ARMS and were negative.
Images of renal biopsy (A) and chest CT (B–F). (A) 2 glomeruli with extracapillary (or crescent) proliferation (periodic acid Schiff). (B–F) Different chest CT slices showing bilateral mixed centrilobular emphysema, with areas of paraseptal involvement and subpleural bullae, mainly in the upper lobes. (F) One of the nodules, measuring 3.1mm (dotted line).
Six months later, after completing induction therapy, the patient achieved clinical remission and began treatment with azathioprine. Respiratory problems included several infections that were managed with oral antibiotics. No significant changes were found on chest CT, and the 3 nodules previously visualized remained stable. Lung tests performed at that time showed DLCO: 46%; KCO: 60%; FEF 25%–75%: 65%; FEV1: 78%; and FEV1/FVC: 76%.
Cases of pulmonary emphysema, some associated with AAT deficiency (AATD), have been reported in patients with AAV.1,2 One clear cause of our patient's pulmonary emphysema was his smoking habit.4 In general, accumulated tobacco consumption correlates with the severity of the lung disease. In the absence of other genetic and/or environmental factors, it is thought very unlikely that lung disease will develop with an exposure of less than 10–15 pack-years, and the only clearly associated factor is a habit of over 40 pack-years.4,5 In our patient, the severity of the emphysema, his age, and tobacco exposure below the limits mentioned above led us to consider other possible causes (such as other toxic substances, and particularly AATD and a deficient genetic allele). However, the contribution of AAV to his pulmonary emphysema cannot be ruled out, and this factor may also explain the deterioration of KCO, despite giving up smoking. The pathogenic link between these 2 factors is not well established. In our case, no clinical evidence of previous diffuse alveolar hemorrhage that might have resulted in emphysema was observed. AAT is an inhibitor of serine proteases, including elastase and PR3, that are found in primary neutrophil granules and are involved in tissue breakdown.6 Tobacco use increases pulmonary levels of metalloproteinase and elastase, released by the alveolar macrophages and neutrophils, respectively, and functional inhibition of AAT.7,8 In vasculitis, ANCA cause degranulation of neutrophils with the consequent release of proteases from their primary granules (PR3 and elastase) (respiratory burst), and also interfere in the formation of PR3-AAT complexes, preventing the neutralization of these proteases.9–12 Therefore, it is possible that in smokers with AAV, protease/antiprotease imbalance in the extracellular fluid results in increased destruction of elastin, a protein matrix essential for maintaining the structural integrity of the lungs, thus contributing to the severity of the pulmonary emphysema. However, the in vivo interaction between PR3, AAT and ANCAs still has not been definitively established. Lastly, the patient's pulmonary nodules, while possibly associated with the ANCA-PR3 vasculitis, were interpreted as nonspecific because they persisted despite remission of the vasculitis.
In short, pulmonary emphysema can coexist with ANCA-associated vasculitis, and the pathogenic contribution of this process, in addition to other clearly associated factors, such as tobacco and AADT, cannot be ruled out.
Please cite this article as: Muray Cases S, Alcázar Fajardo C, Cabezuelo Romero JB. Enfisema pulmonar severo en un paciente joven con una vasculitis asociada a anticuerpos anticitoplasma de neutrófilo tipo proteinasa-3 (ANCA-PR3). Arch Bronconeumol. 2018;54:397–399