Galcanezumab is a humanized monoclonal antibody targeting the calcitonin gene-related peptide approved for preventing chronic migraine in adults [1]. As a potent vasodilator, its inhibition could theoretically cause cardiovascular events, including pulmonary hypertension (PH). Such effects were not observed in clinical trials or post-marketing studies [2].
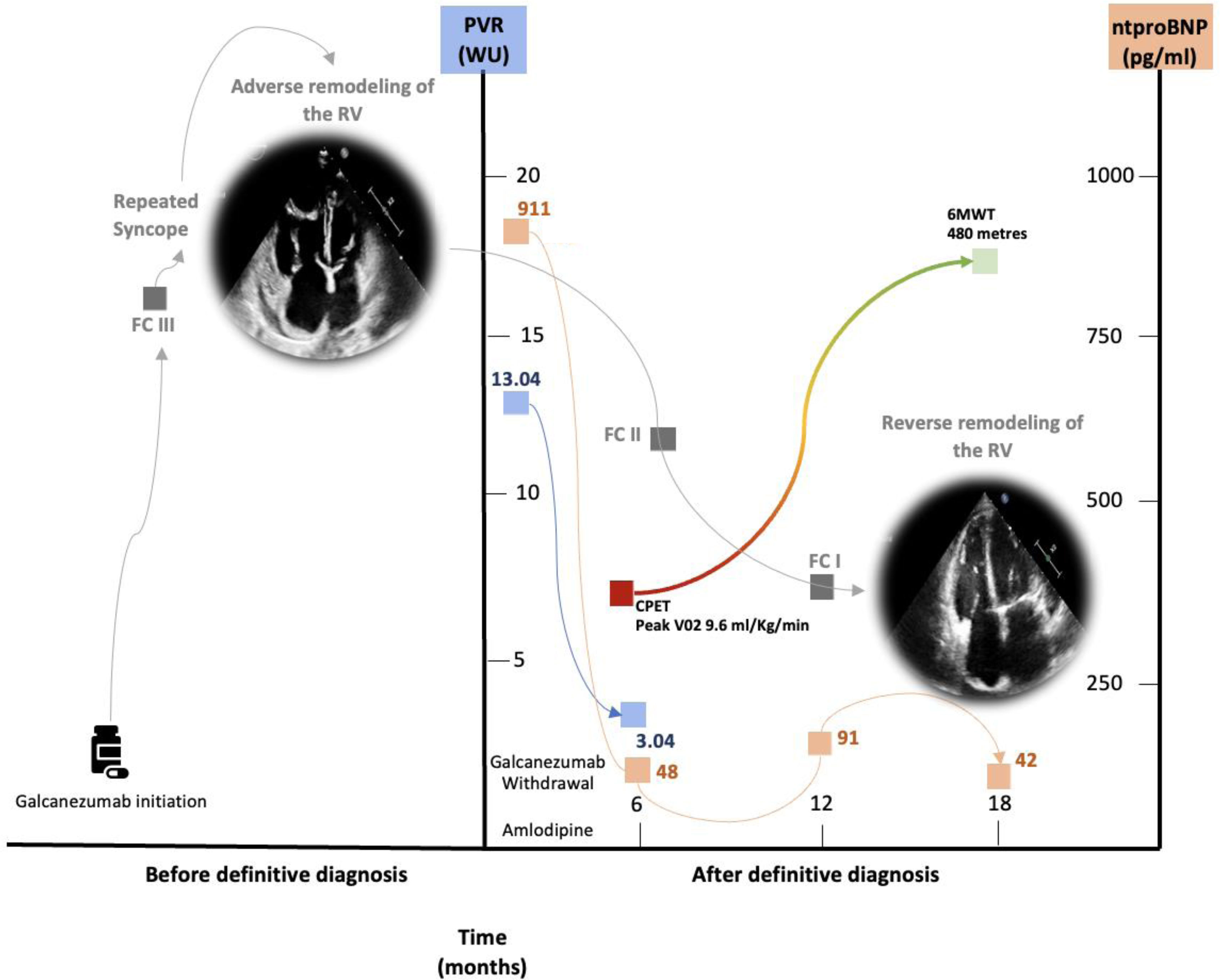
We present the case of a 65-year-old woman with idiopathic pulmonary arterial hypertension (iPAH) with a chronic, sustained response to calcium channel blockers (CCBs) treated with galcanezumab for severe, refractory chronic migraine. She had a long-standing history of migraine since childhood, initially managed with NSAIDs and triptans. As migraines became chronic, she underwent multiple preventive treatments (including amitriptyline, nebivolol, flunarizine, desvenlafaxine, valproate, lamotrigine, topiramate, zonisamide, and botulinum toxin) without benefit. In February 2022, she began galcanezumab in monotherapy (240mg loading dose, and 120mg monthly). Soon after, she developed progressive dyspnea and had two syncopal episodes. Respiratory insufficiency was not documented, and pulmonary function tests were unremarkable. The initial NT-proBNP was of 900pg/mL. An echocardiogram showed right heart dilation and high pulmonary pressures (>100mmHg). Right heart catheterization (RHC) confirmed severe precapillary PH (mPAP 38mmHg, CO 2.53L/min, PVR 13.04 Wood units). A vasodilator test with nitric oxide led to a significant drop in mPAP (to 22mmHg). After excluding other PH etiologies, galcanezumab was withdrawn. The patient was diagnosed with iPAH with acute vasoreactivity and treated with high-dose amlodipine. Genetic testing was negative. A follow-up RHC after six months confirmed a sustained hemodynamic response. The patient remains asymptomatic three years after the diagnosis, with normal NT-proBNP levels (Fig. 1). Her migraine is also well-controlled on amlodipine monotherapy.
Clinical summary of the patient, including clinical presentation, haemodynamic parameters, echocardiographic findings, functional assessments, and cardiac biomarkers at diagnosis and during follow-up. At diagnosis, functional capacity was evaluated by cardiopulmonary exercise testing (CPET) with a cycle ergometer, and by the 6-minute walk test (6MWT) during follow-up. Functional capacity changes (red to green), pulmonary vascular resistance (blue), and NT-proBNP levels (salmon pink) are illustrated in the figure. CPET, cardiopulmonary exercise testing; FC, functional class; PVR, pulmonary vascular resistance; RV, right ventricle; 6MWT, 6-minute walk test.
This is, to our knowledge, the first reported case of iPAH potentially associated with galcanezumab. The temporal relationship, improvement following discontinuation, and biological plausibility suggest a possible causal link. Galcanezumab has not been flagged in pharmacovigilance databases like VigiBase, but other agents with vasoconstrictive properties have [3]. Interestingly, an Australian consensus on galcanezumab identified three patients with unexplained dyspnoea, although PH was not considered as a plausible cause in these cases by the authors of this public report [4]. Further studies are needed to explore whether galcanezumab can trigger or induce PH in susceptible individuals. Post-marketing surveillance remains essential to detect rare but severe adverse drug reactions.
CRediT authorship contribution statementSubstantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: ACU, DTA, PES.
Drafting the work or revising it critically for important intellectual content: ACU, DTA, PES.
Final approval of the version to be published: ACU, DTA, PES.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: ACU, DTA, PES.
Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that no artificial intelligence applications have been used for this work.
FundingACU holds a research grant from the Instituto de Salud Carlos III (ISCIII), Ministry of Science and Innovation, Spanish Government (JR23/00071).
Conflict of interestACU has received lecture fees from Janssen, Ferrer, AOP Orphan, Gossamer Bio, and MSD. PES has served on advisory boards for Janssen, Gossamer Bio, and Liquidia.
DTA has received lecture fees from Lilly, Lundbeck, and Teva.
PES has received lecture fees from Janssen, Ferrer, AOP Orphan, Gossamer Bio, and MSD. PES has served on advisory boards for Janssen, Gossamer Bio, and MSD.