The therapeutic approach for metastatic malignant pleural effusion depends on the patient's life expectancy. Can survival be accurately estimated in these patients using a risk-prediction model?
MethodsA prospective, single-center study was conducted to examine the prognostic value of pre-established variables (multivariate Cox model). Subsequently, a prognostic score was developed and validated. The inclusion period was 11 years long. Follow-up was conducted until death or for a minimum of 12 months.
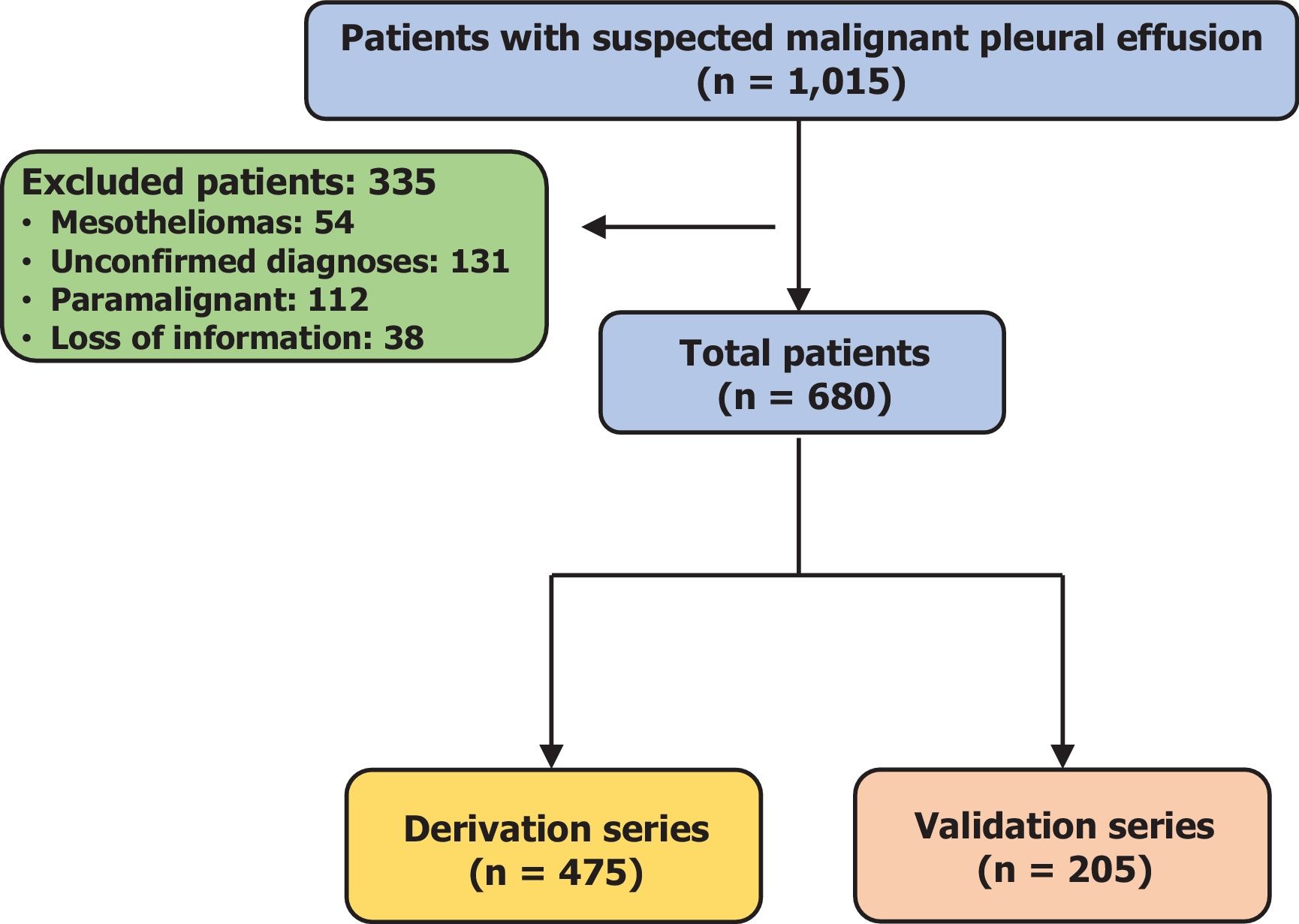
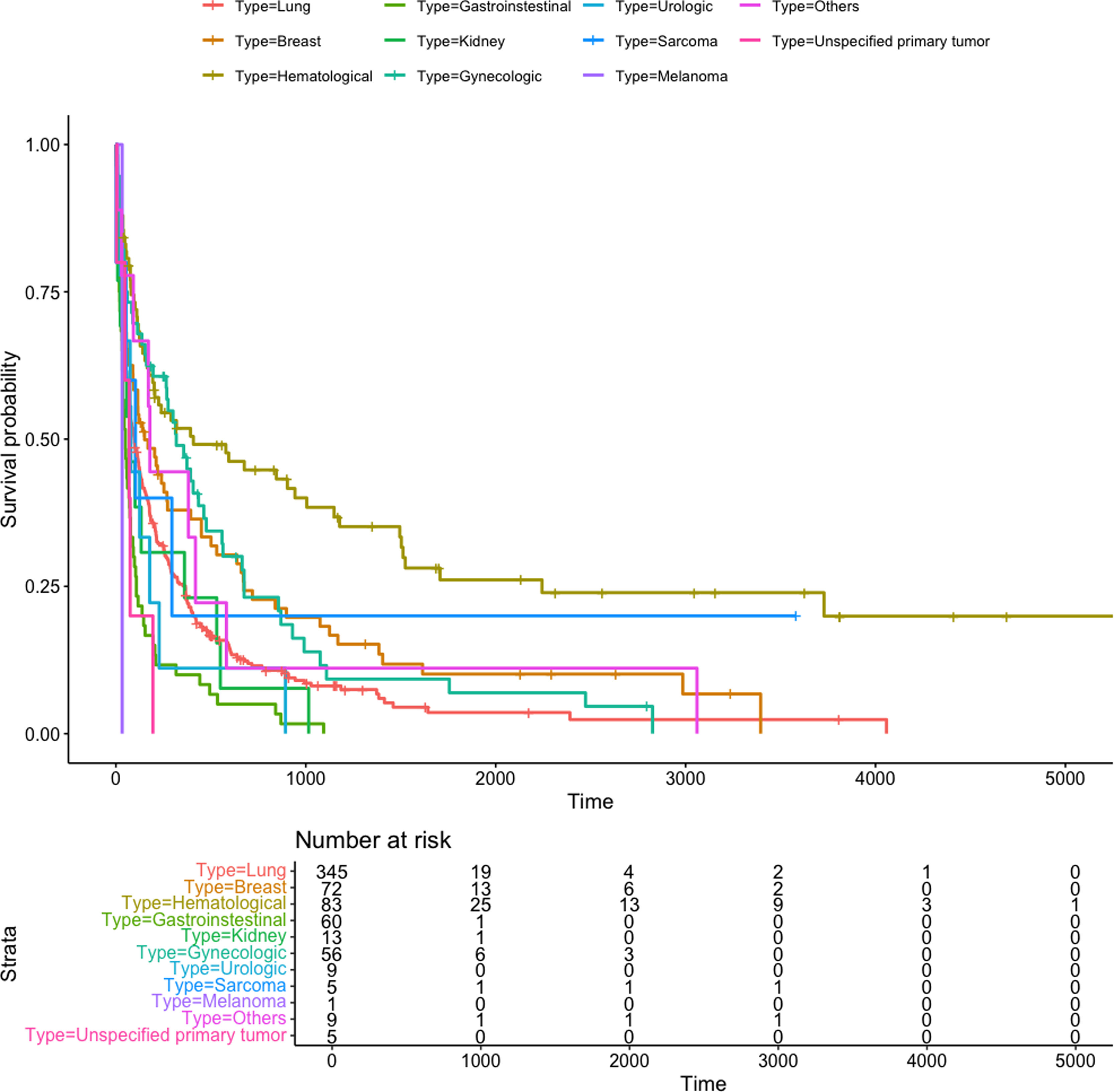
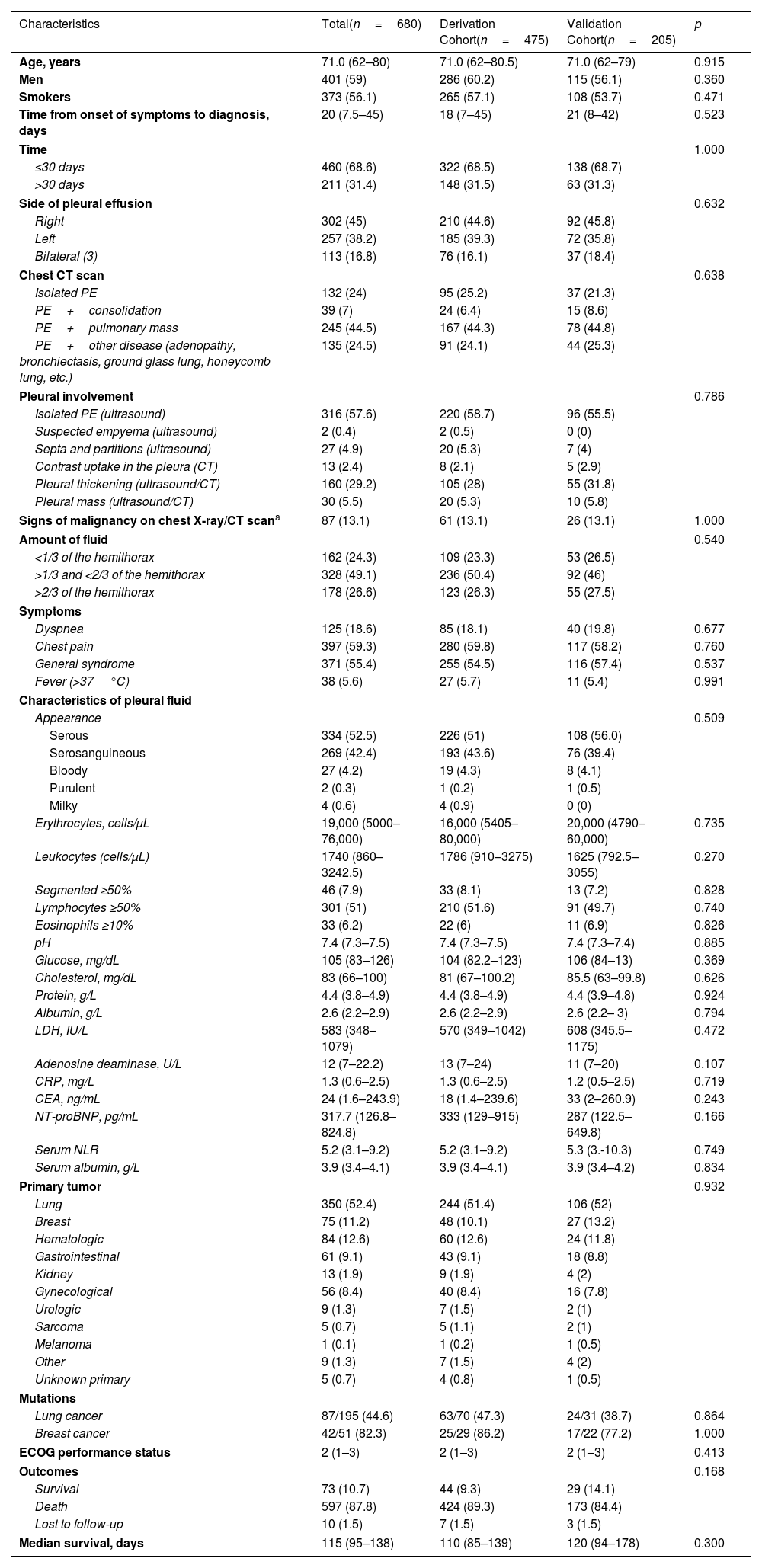
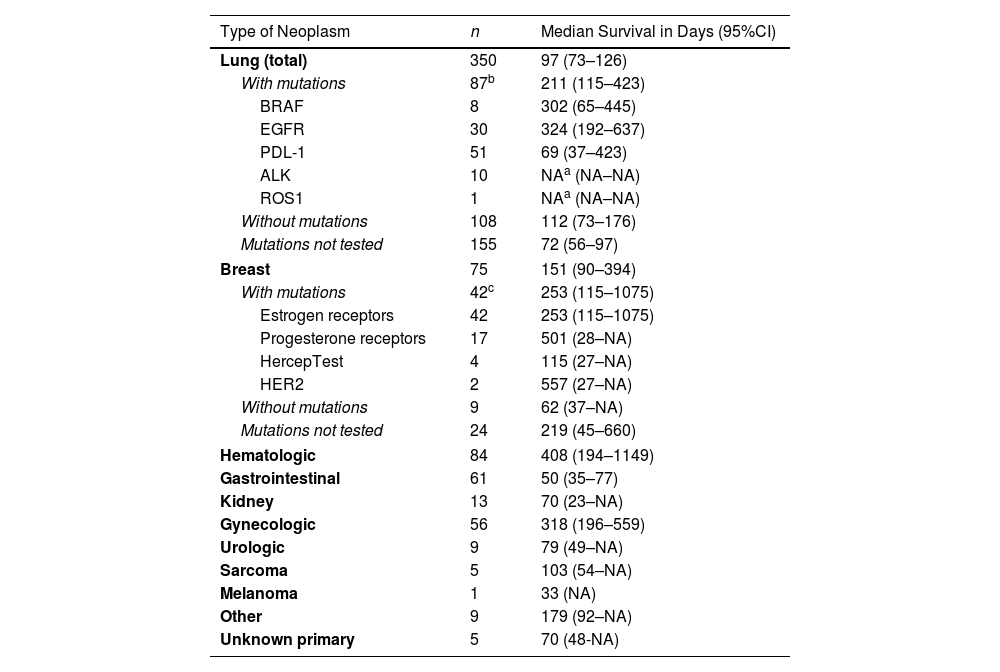
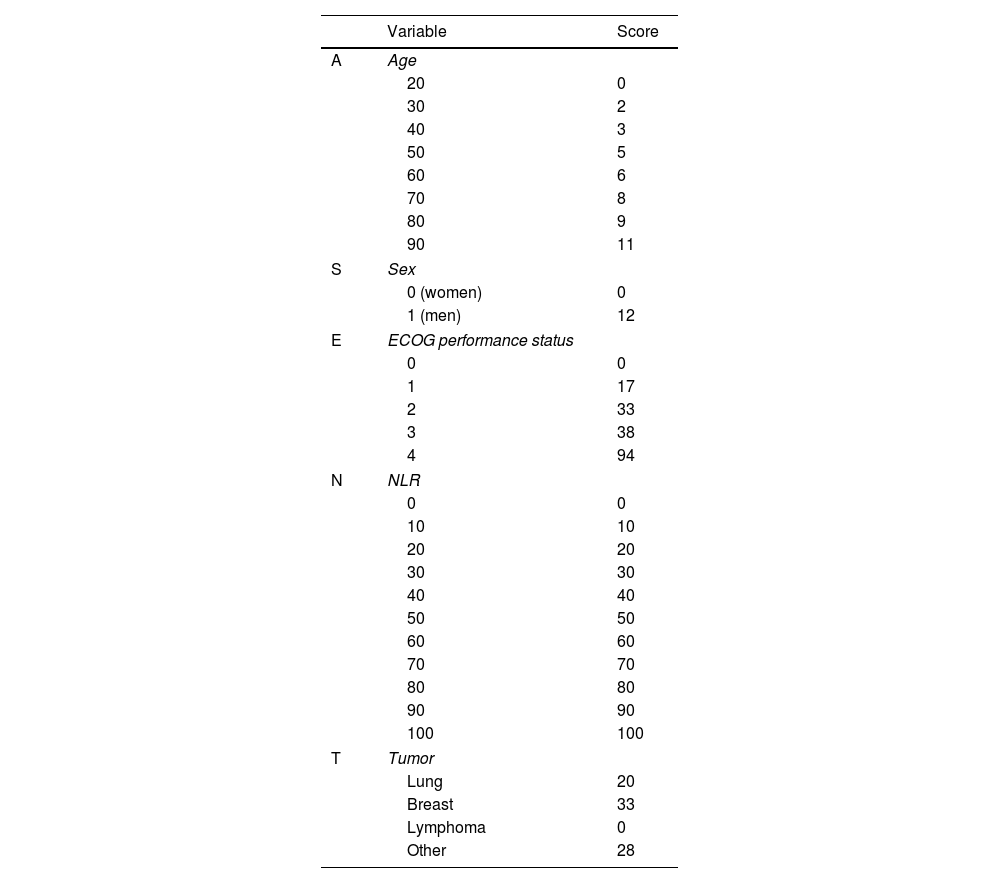
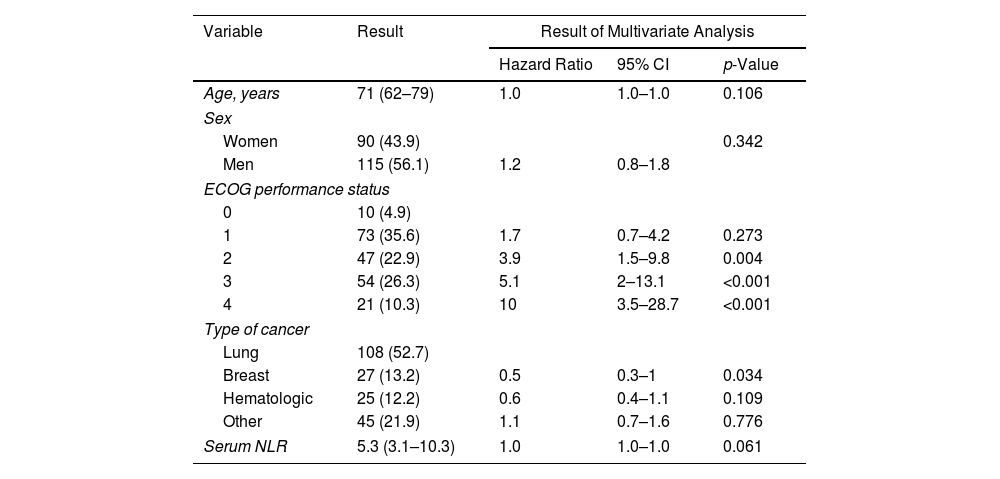
ResultsThe derivation and validation cohorts included 475 and 205 patients, respectively. The prognostic score GASENT (Galicia, Age, Sex, ECOG-PS, Neutrophil/lymphocyte ratio, and Tumor type) was derived from the multivariate analysis of survival.
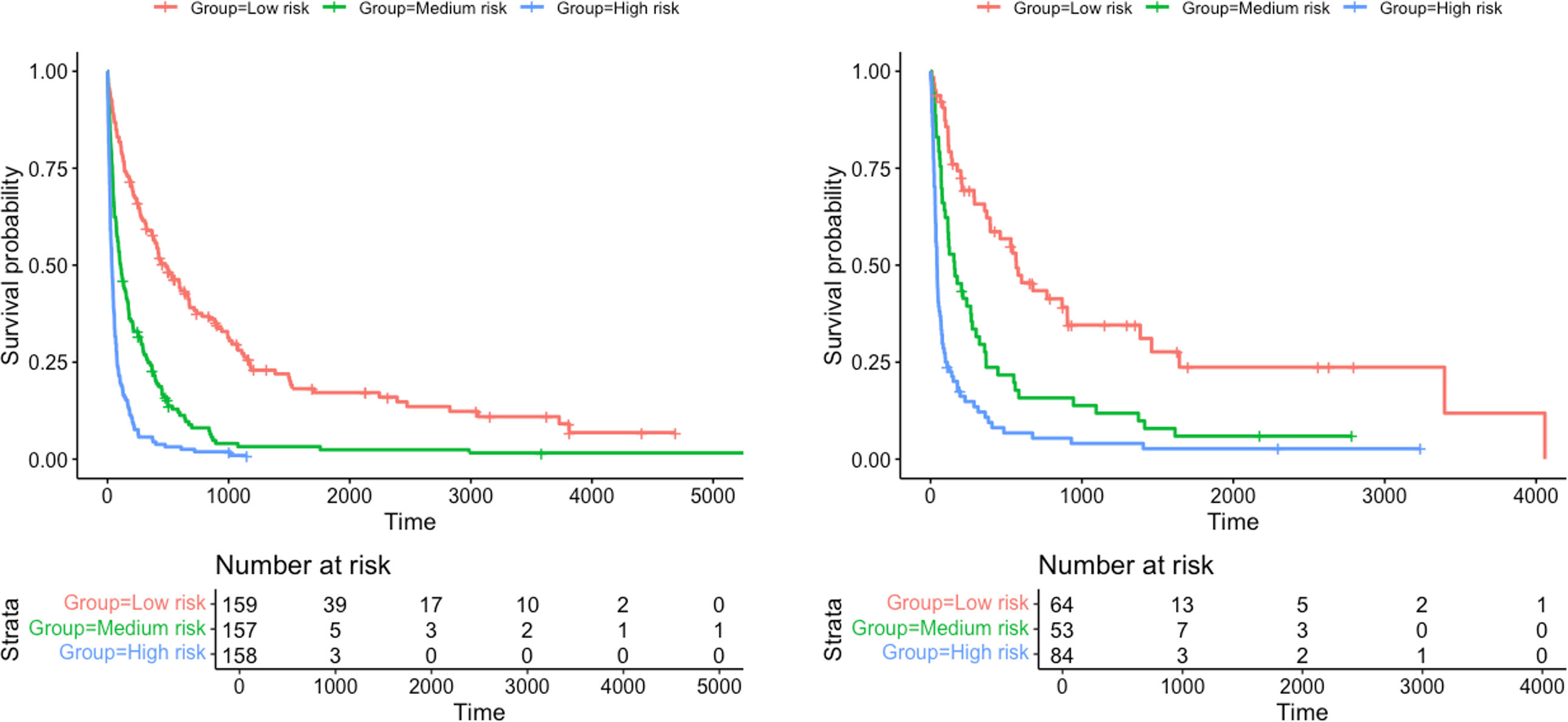
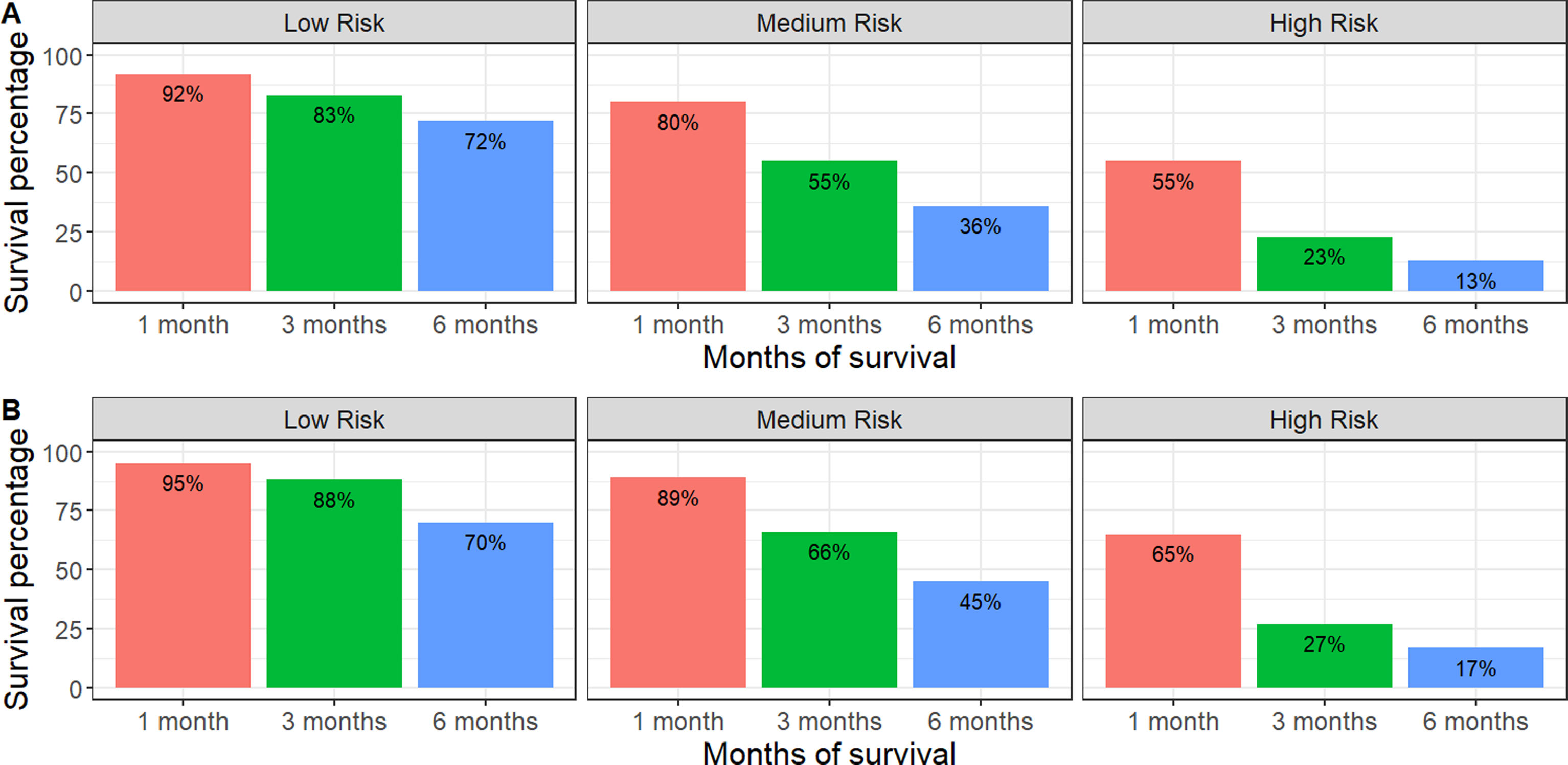
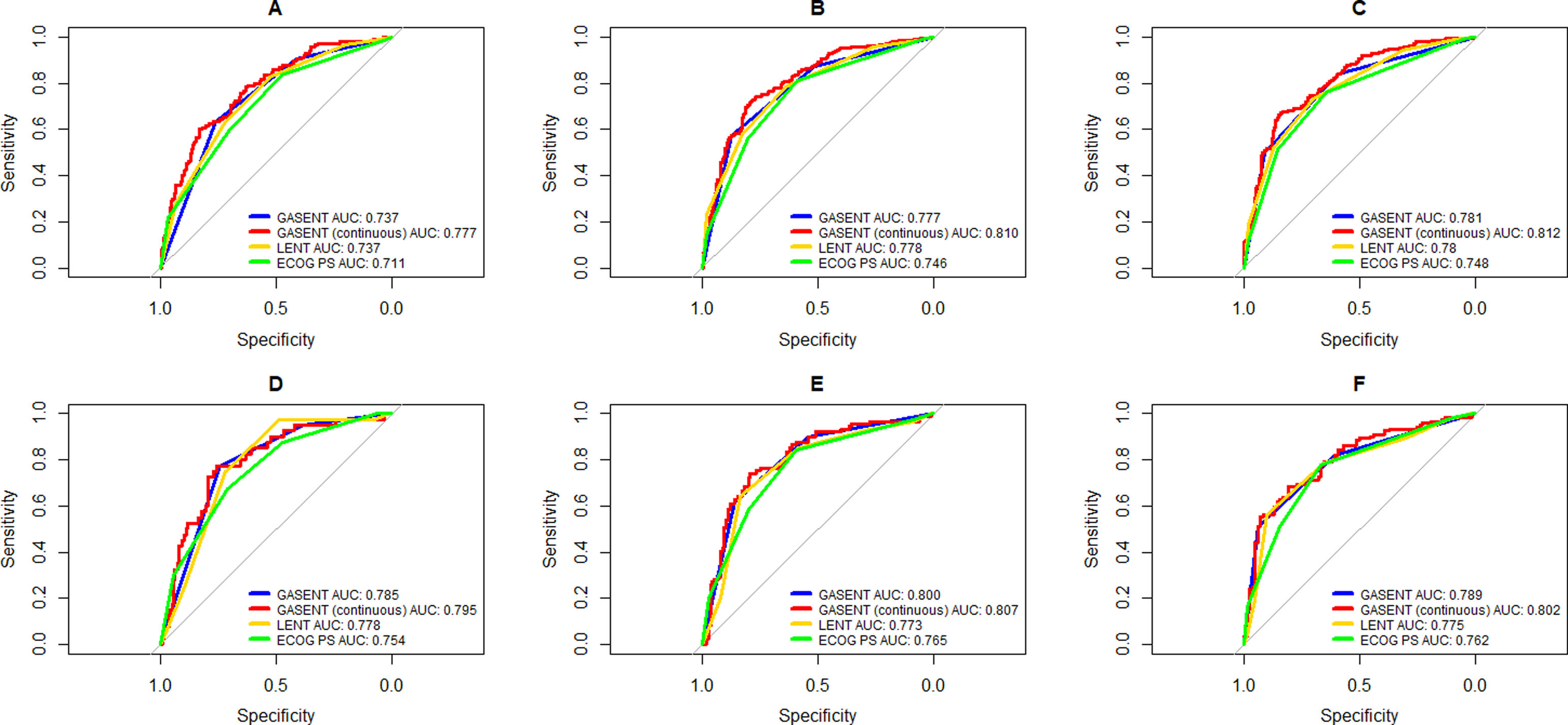
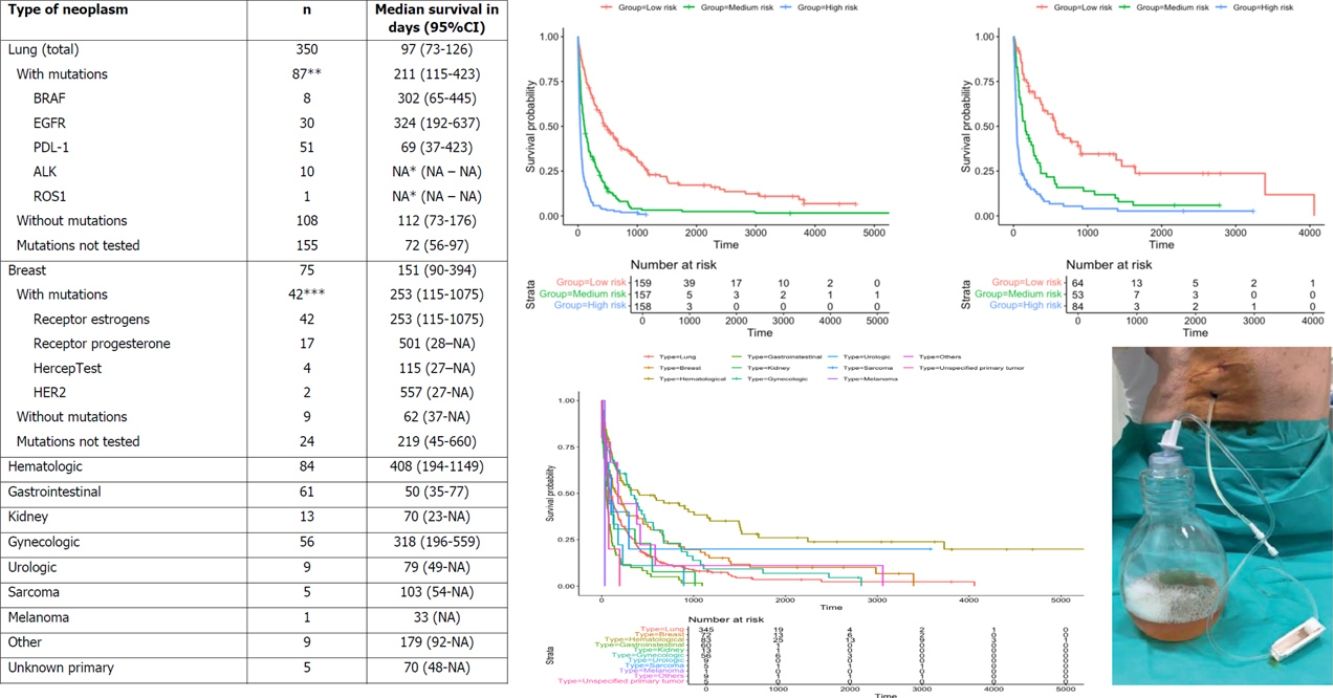
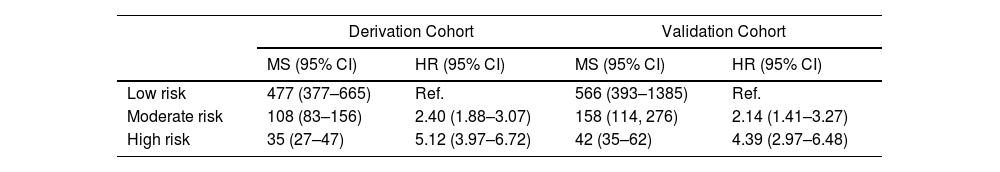
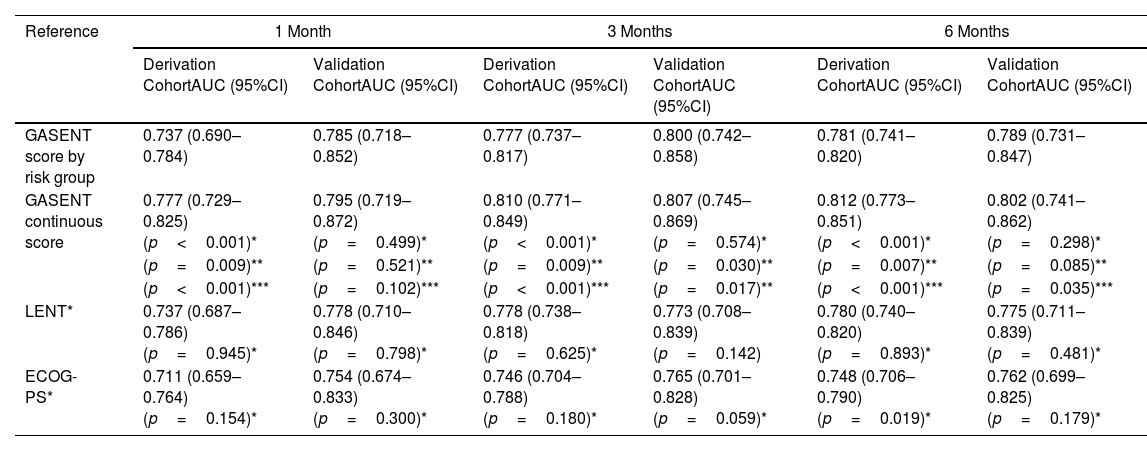
Categorization of patients in the derivation cohort into low-, moderate-, or high-risk yielded median survival times of 477 days (377–665; n=159), 108 days (83–156; n=158), and 35 days (27–47; n=158), respectively. Survival rates at 1, 3, and 6 months were 92%, 83%, and 72%, respectively, for the low-risk group; 80%, 55%, and 36%, respectively, for the moderate-risk group; and 55%, 23%, and 13%, respectively, for the high-risk group. The analysis of areas under the curve revealed that the GASENT model was superior to the LENT score as a survival predictive model at 1 (0.777 vs. 0.737; p=0.009), 3 (0.810 vs. 0.778; p=0.009), and 6 months (0.812 vs. 0.780; p=0.007).
ConclusionsThe GASENT predictive model estimates survival in patients with metastatic malignant effusions with significantly greater accuracy than the scores categorizing patients by risk groups.