The study aimed to identify perioperative variables associated with unplanned reoperation (UR) following anatomical pulmonary resection for lung cancer and investigate its impact on long-term prognostic outcomes.
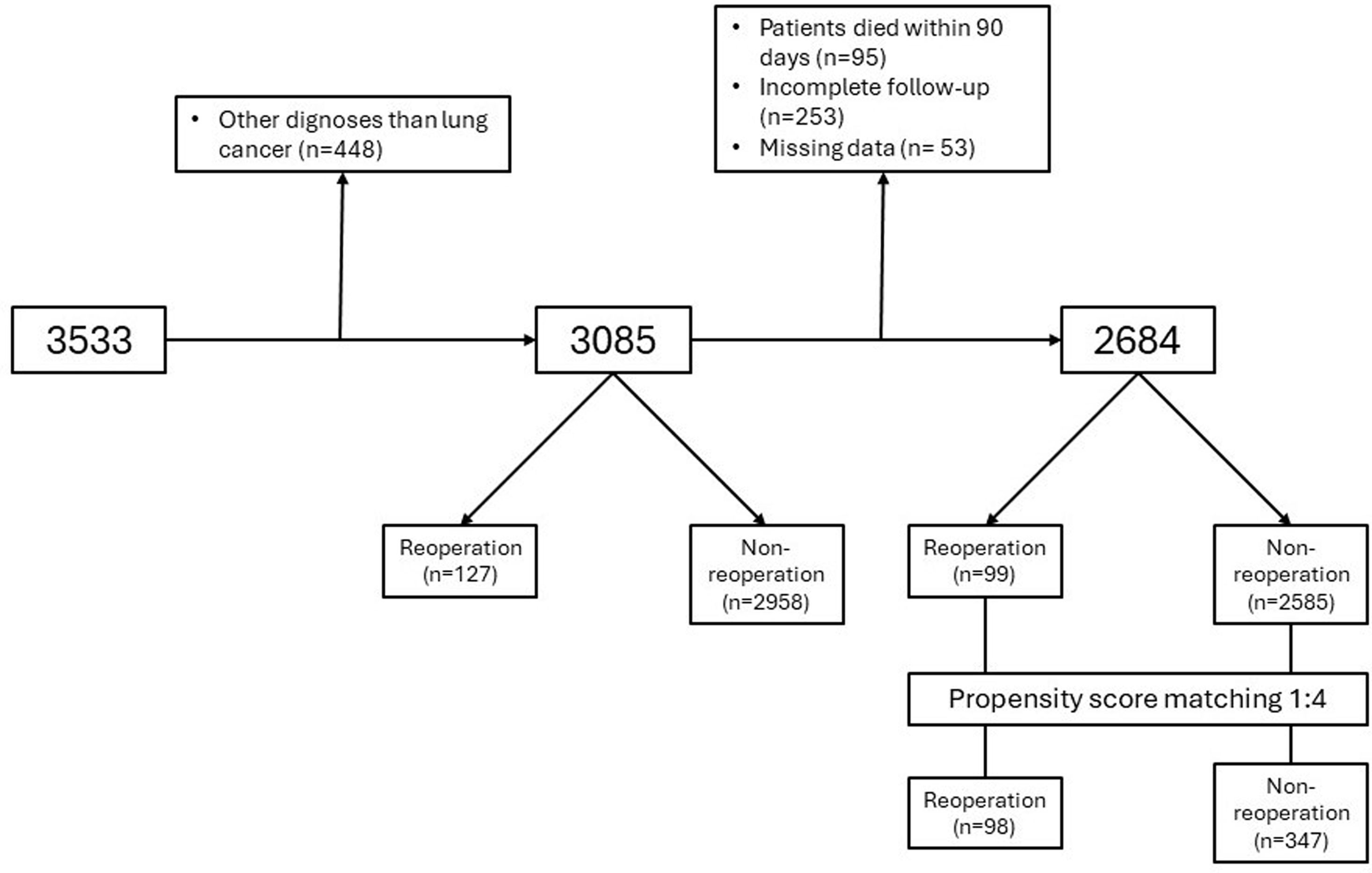
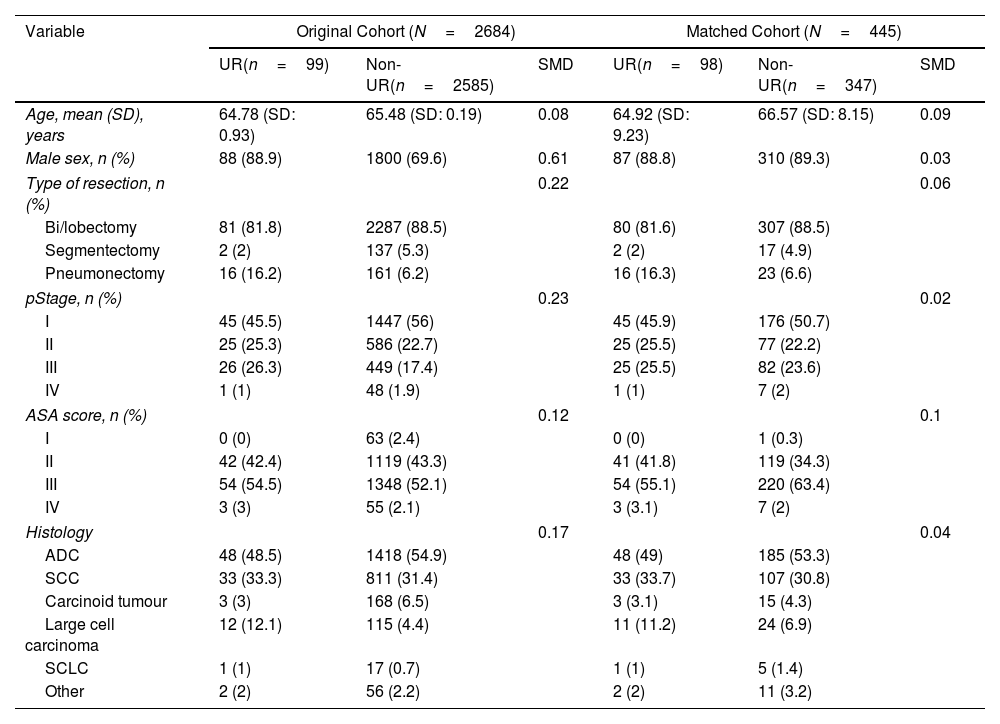
MethodsThe records of patients who underwent anatomical pulmonary resection for lung cancer from December 2016 to March 2018 within a nationwide prospective registry were reviewed. Multivariable logistic regression analyses were performed to find the risk factors for UR. The short-term outcomes were compared, and the adjusted odds ratios for in-hospital and 90-day mortality were calculated. The prognostic value of UR for overall survival (OS) and disease-free survival (DFS) was assessed using the Kaplan–Meier method and log-rank test after propensity score matching for balancing baseline confounders.
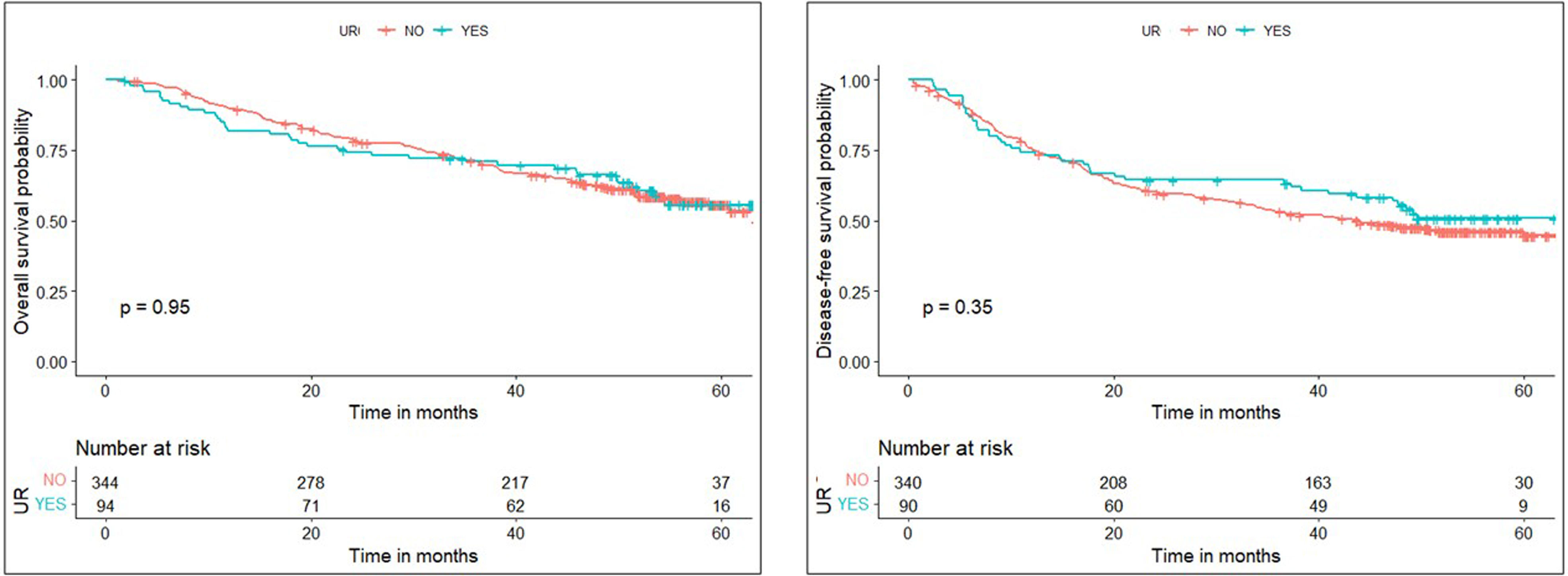
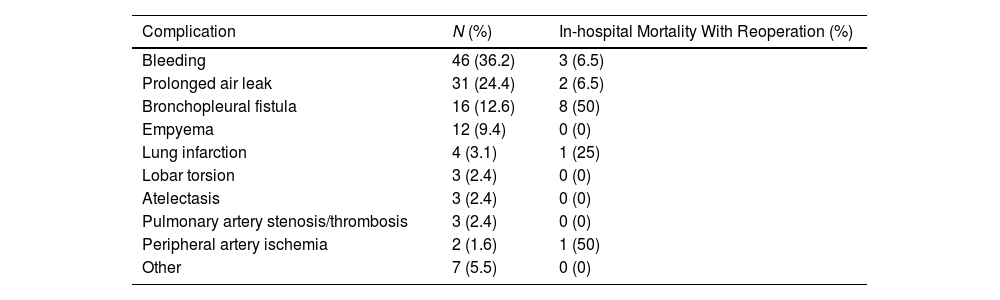
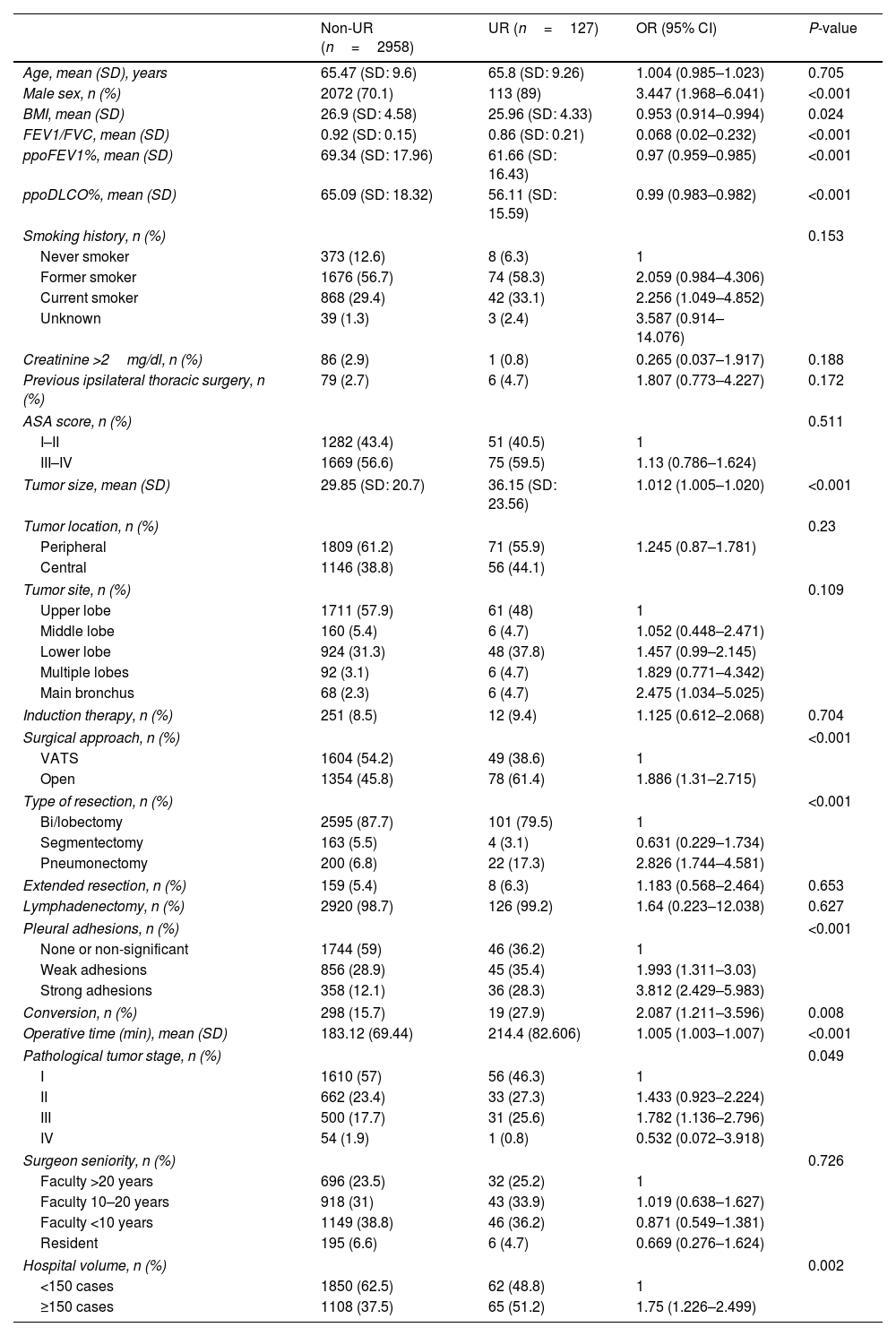
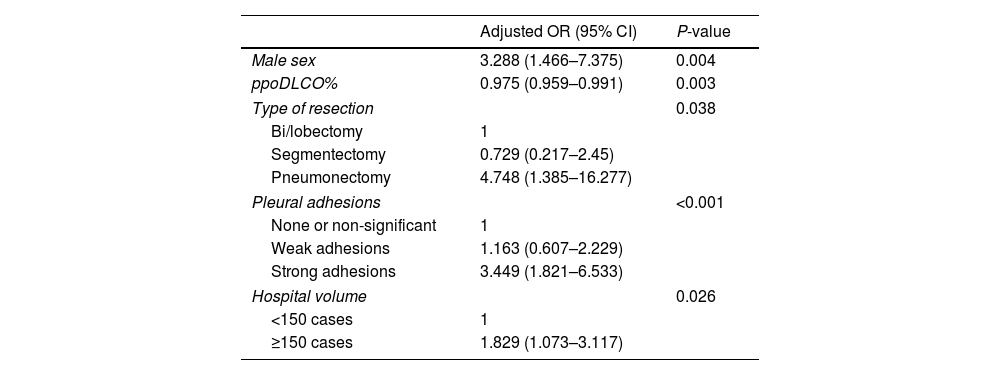
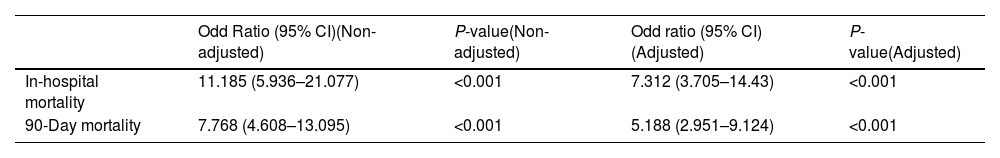
ResultsData from 3085 patients were examined, revealing a UR incidence of 4.12%. Multivariable logistic regression analyses revealed that male gender (OR=3.288, P=0.004), ppoDLCO% (OR=0.975, P=0.003), pneumonectomy (OR=4.748, P=0.038), strong pleural adhesions (OR=3.449, P<0.001) and hospital volume ≥150 cases (OR=1.75, P=0.026) were independently associated with UR. Risk of in-hospital and 90-day mortality was higher in UR cases (adjusted OR=7.312, P<0.001, and OR=5.188, P<0.001, respectively). Ninety-eight UR and 347 matched non-UR cases were included in the long-term follow-up analysis. The median follow-up time was 50.4 months. No significant differences were found in OS, and DFS between groups (log rank P=0.953 and P=0.352, respectively).
ConclusionMale gender, ppoDLCO%, pneumonectomy, strong pleural adhesions, and surgical unit workload were all independently associated with UR. UR was associated with an increased perioperative mortality, but not with a higher long-term mortality.