We aim to describe the changes in prevalence and risk factors associated to chronic obstructive pulmonary disease (COPD) in Spain, comparing three population-based studies conducted in three timepoints.
MethodsWe compared participants from IBERPOC conducted in 1997, EPISCAN conducted in 2007 and EPISCAN II in 2017. COPD was defined as a postbronchodilator FEV1/FVC (forced expiratory volume in 1s/forced vital capacity) ratio <0.70, according to GOLD criteria; subsequently, also as the FEV1/FVC below the lower limit of normal (LLN).
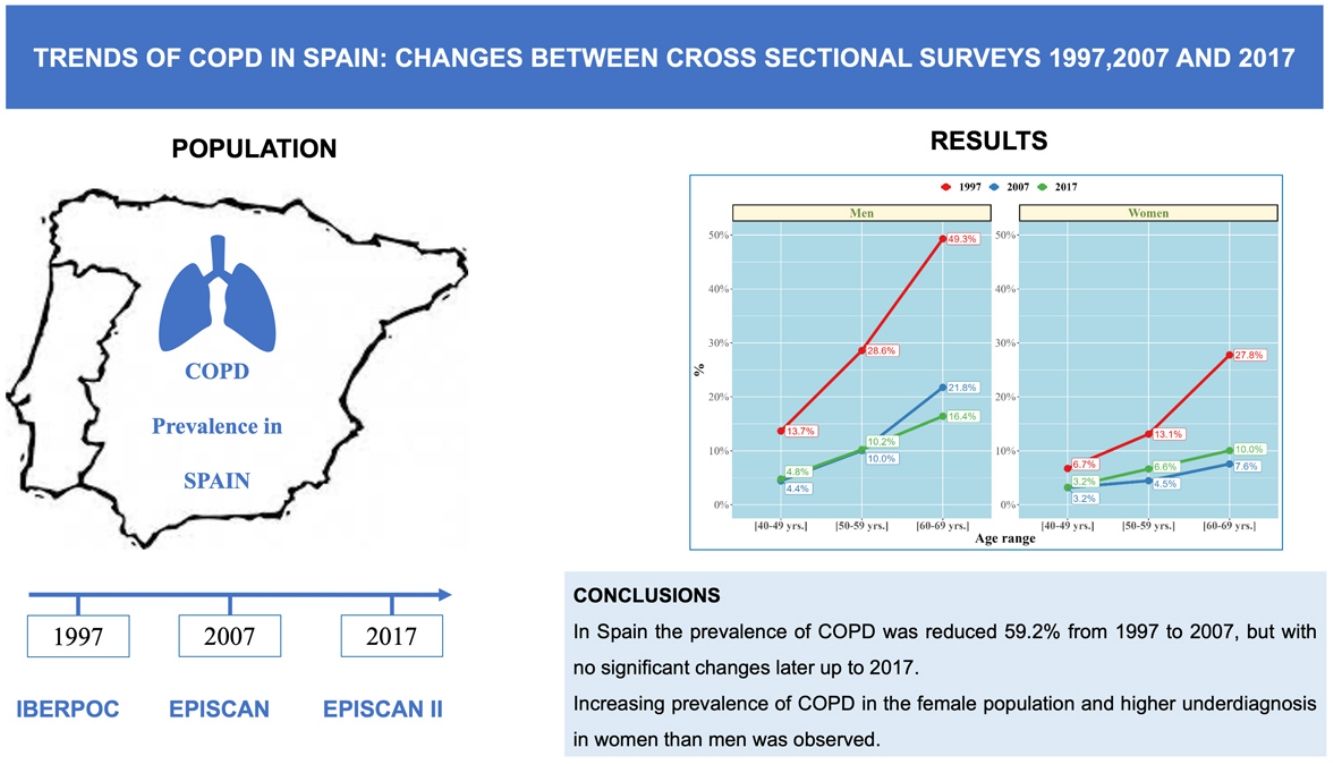
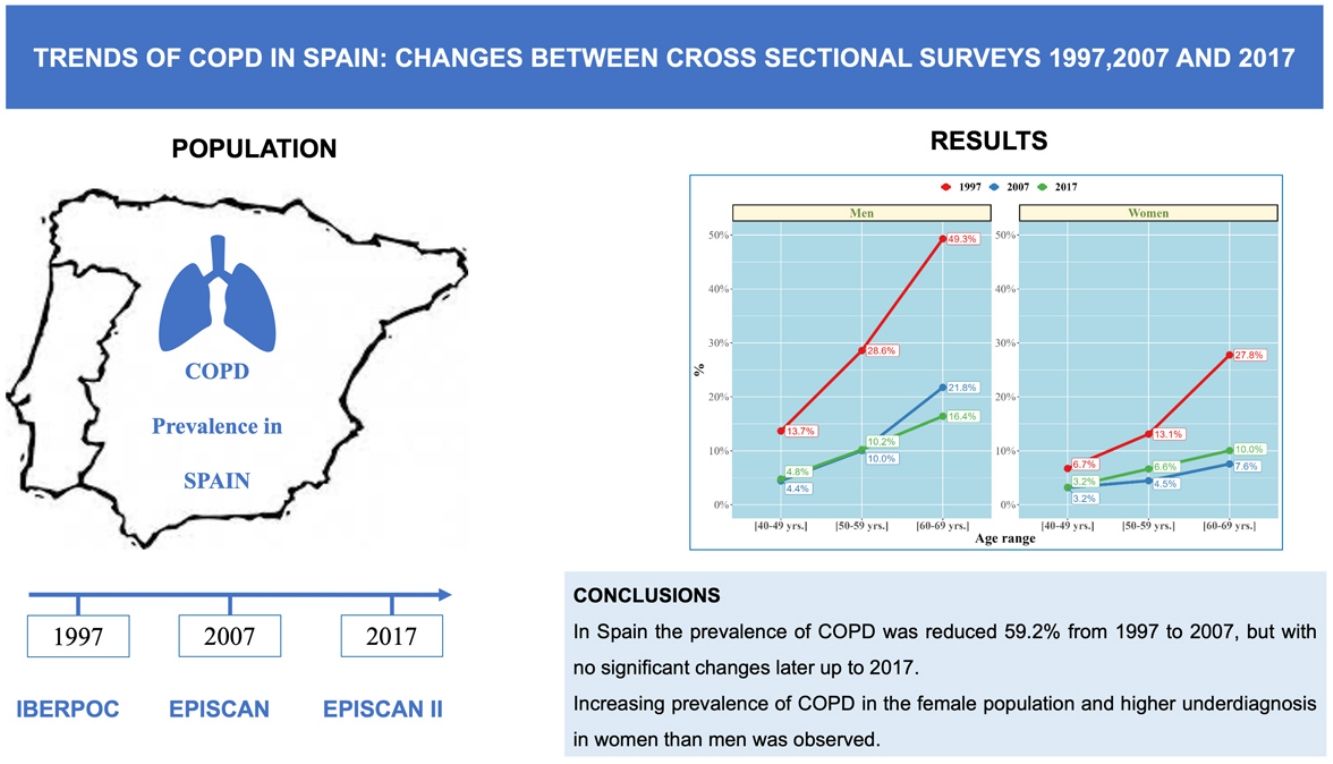
ResultsCOPD prevalence in the population between 40 and 69 years decreased from 21.6% (95% CI 20.7%–23.2%) in 1997 to 8.8% (95% CI 8.2%–9.5%) in 2017, a 59.2% decline (p<0.001).
In 2007, the prevalence was 7.7% (95% CI 6.8%–8.7%) with an upward trend of 1.1 percentage points in 2017 (p=0.073). Overall COPD prevalence decreased in men and women, although a significant increase was observed in the last decade in females (p<0.05). Current smokers significantly increased in the last decades (25.4% in 1997, 29.1% in 2007 and 23.4% in 2017; p<0.001). Regrettably, COPD underdiagnosis was constantly high, 77.6% in 1997, 78.4% in 2007, and to 78.2% in 2017 (p=0.95), higher in younger ages (40–49 yrs and 50–59 yrs) and also higher in women than in men in all three studies (p<0.05).
ConclusionsWe report a significant reduction of 59.2% in the prevalence of COPD in Spain from 1997 to 2017 in subjects aged 40–69 years. Our study highlights the significant underdiagnosis of COPD, particularly sustained in women and younger populations.
Chronic obstructive pulmonary disease (COPD) is one of the main contributors to the global disease burden, representing a significant health problem due to its high mortality and morbidity worldwide.1 Despite this, it is still an underdiagnosed and undertreated disease, often diagnosed late in life or with advanced severity.
Because of the significant impact on morbidity, mortality, and related healthcare spending, it is essential to determine the prevalence of COPD and its risk factors periodically. A number of epidemiological studies on the distribution of COPD are available, albeit with major differences in the methodologies used, the diagnostic criteria, and the geographical setting among others, being difficult to establish comparisons between studies.2–4 Therefore, data have to be re-analyzed and results translated to identify changes in time.
In Spain, the prevalence of COPD has been evaluated through three nation-wide epidemiological studies in the last twenty years. The Estudio epidemiológico de la EPOC (IBERPOC)5 conducted in 1997 found a prevalence of 9.1% in the general Spanish population aged 40–69 years, using the 1995 European Respiratory Society (ERS) guidelines to define COPD.6 The Epidemiologic Study of COPD in Spain (EPISCAN),7 conducted in 2007, found a prevalence of a postbronchodilator forced expiratory volume in 1s/forced vital capacity (FEV1/FVC) ratio <0.70 of 10.2% in the general Spanish population aged 40–80 years. Finally, the second Epidemiologic Study of COPD in Spain (EPISCAN II), conducted in 2017 with a very similar methodology as the previous one, reported a prevalence of 11. 8% in the general Spanish population aged 40 years or older.8 In these studies, high rates of underdiagnosis of COPD were described: 78% in 1997, 73% in 2007 and 74.7% in 2017.
Monitoring trends of COPD prevalence and its associated risk factors can be beneficial before implementing preventive initiatives and control measures, such as to reduce underdiagnosis.
In the current study, we use patient-level data from three cross-sectional studies conducted in 1997, 2007 and 2017 to determine changes in COPD prevalence and risk factors associated in the general Spanish population from 1997 to 2017.
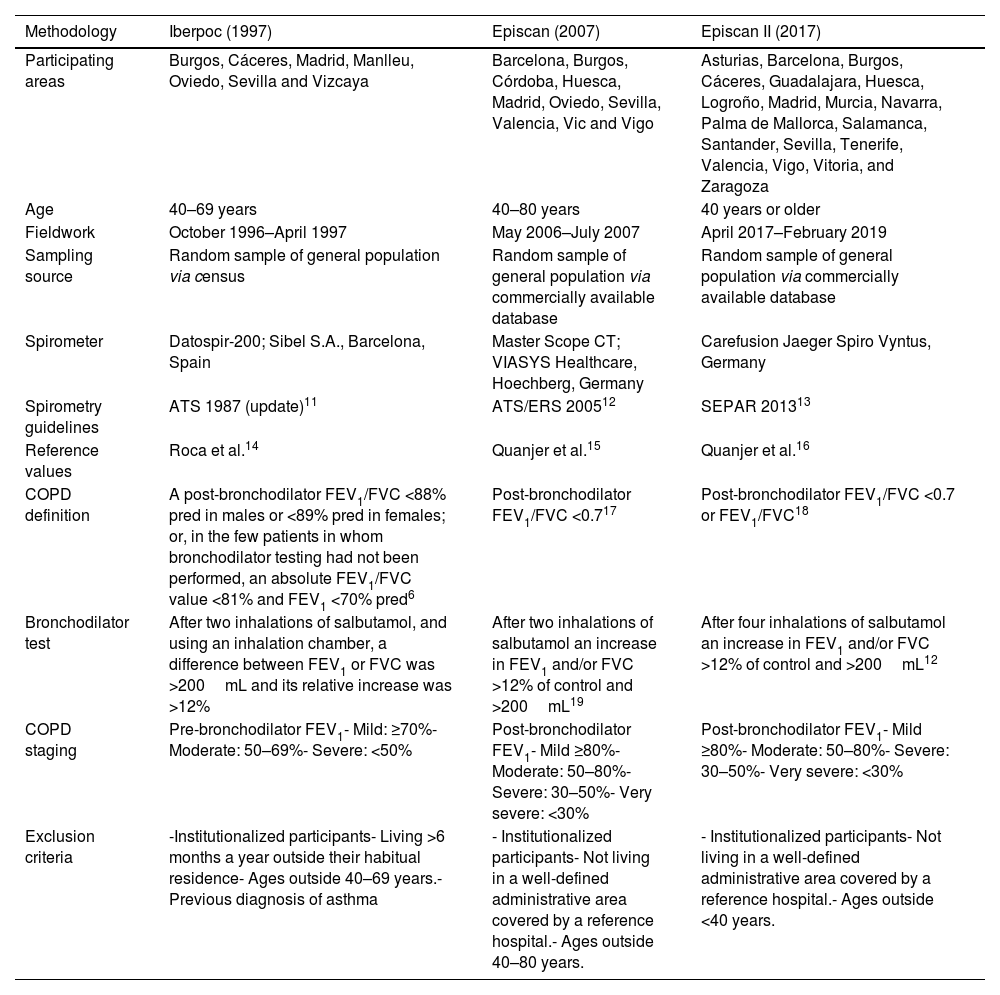
Materials and MethodsStudy PopulationFor this analysis, we used data from all 17,270 participants from IBERPOC,5 EPISCAN7 and EPISCAN II.8 We applied a repeated cross-sectional study design to compare the COPD prevalence in these studies in 1997, 2007 and 2017. The methodology of the three studies has been detailed elsewhere,5,9,10 and their main differences and similarities are compared in Table 1.
Comparison of Study Designs Used in 1997, 2007 and 2017.
| Methodology | Iberpoc (1997) | Episcan (2007) | Episcan II (2017) |
|---|---|---|---|
| Participating areas | Burgos, Cáceres, Madrid, Manlleu, Oviedo, Sevilla and Vizcaya | Barcelona, Burgos, Córdoba, Huesca, Madrid, Oviedo, Sevilla, Valencia, Vic and Vigo | Asturias, Barcelona, Burgos, Cáceres, Guadalajara, Huesca, Logroño, Madrid, Murcia, Navarra, Palma de Mallorca, Salamanca, Santander, Sevilla, Tenerife, Valencia, Vigo, Vitoria, and Zaragoza |
| Age | 40–69 years | 40–80 years | 40 years or older |
| Fieldwork | October 1996–April 1997 | May 2006–July 2007 | April 2017–February 2019 |
| Sampling source | Random sample of general population via census | Random sample of general population via commercially available database | Random sample of general population via commercially available database |
| Spirometer | Datospir-200; Sibel S.A., Barcelona, Spain | Master Scope CT; VIASYS Healthcare, Hoechberg, Germany | Carefusion Jaeger Spiro Vyntus, Germany |
| Spirometry guidelines | ATS 1987 (update)11 | ATS/ERS 200512 | SEPAR 201313 |
| Reference values | Roca et al.14 | Quanjer et al.15 | Quanjer et al.16 |
| COPD definition | A post-bronchodilator FEV1/FVC <88% pred in males or <89% pred in females; or, in the few patients in whom bronchodilator testing had not been performed, an absolute FEV1/FVC value <81% and FEV1 <70% pred6 | Post-bronchodilator FEV1/FVC <0.717 | Post-bronchodilator FEV1/FVC <0.7 or FEV1/FVC18 |
| Bronchodilator test | After two inhalations of salbutamol, and using an inhalation chamber, a difference between FEV1 or FVC was >200mL and its relative increase was >12% | After two inhalations of salbutamol an increase in FEV1 and/or FVC >12% of control and >200mL19 | After four inhalations of salbutamol an increase in FEV1 and/or FVC >12% of control and >200mL12 |
| COPD staging | Pre-bronchodilator FEV1- Mild: ≥70%- Moderate: 50–69%- Severe: <50% | Post-bronchodilator FEV1- Mild ≥80%- Moderate: 50–80%- Severe: 30–50%- Very severe: <30% | Post-bronchodilator FEV1- Mild ≥80%- Moderate: 50–80%- Severe: 30–50%- Very severe: <30% |
| Exclusion criteria | -Institutionalized participants- Living >6 months a year outside their habitual residence- Ages outside 40–69 years.- Previous diagnosis of asthma | - Institutionalized participants- Not living in a well-defined administrative area covered by a reference hospital.- Ages outside 40–80 years. | - Institutionalized participants- Not living in a well-defined administrative area covered by a reference hospital.- Ages outside <40 years. |
COPD: chronic obstructive pulmonary disease; IBERPOC: Estudio epidemiológico de la EPOC en España; EPI-SCAN: Epidemiologic Study of COPD in Spain; ATS: American Thoracic Society; ERS: European Respiratory Society; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1s; FVC: forced expiratory volume; % pred: % predicted; LLN: lower limit of normal.
For the purpose of our study, the current GOLD recommendations20 have been used to define and stage COPD, using the definition of a post-bronchodilator spirometry ratio of FEV1 to FVC below 0.70, regardless of the symptoms of the subjects. IBERPOC prevalence was recalculated following this criterion. We also described COPD prevalence by the ratio FEV1/FVC of less than the lower limit of normal (LLN; FEV1/FVC<LLN)18 and for COPD staging, IBERPOC original results were reclassified using EPISCAN and EPISCAN II criteria.
All participants with invalid spirometry (spirometries with quality grades D and higher) were excluded from the analyses.
MeasurementsAny previous diagnosis of COPD, emphysema, chronic bronchitis and asthma were considered when the interviewee gave a positive answer to these previous diagnoses. Questions on previous medical diagnosis compatible with COPD, and on prescribed respiratory treatments, were the same/similar in all three surveys and they were used to determine changes in underdiagnosis and undertreatment.
Questionnaires were administered by trained staff and included information on respiratory symptoms, respiratory diagnoses and risk factors for COPD. Underdiagnosis of COPD was considered when participants had a postbronchodilator FEV1/FVC <0.7 but were not previously diagnosed with COPD by a healthcare professional.
Spirometry was conducted according to standardized procedures as indicated by SEPAR guidance13 by trained technicians. Each spirometry was reviewed, and only spirograms that met acceptability and reproducibility criteria were included.
The three studies were approved by the local ethics committees, and all participants provided written informed consent to participate in the studies.
Statistical AnalysisA descriptive and comparative analysis of sociodemographic and clinical variables between the three studies was performed. EPISCAN and EPISCAN II samples were recalculated including all subjects and defining the same age group (40–69 yrs) as the IBERPOC study. Results are expressed as mean±standard deviation (SD) for quantitative variables and percentage for qualitative variables. Prevalences are presented as percentages and their 95% confidence intervals.
The Shapiro–W Kolmogorov–Smirnov test was carried out to test for normality of continuous variables. Homoscedasticity was tested using Levene's test. A parametric test (t-test) was performed when distributions were normal and homoscedastic. In the case that at least one of these two assumptions was not fulfilled, a non-parametric test (Mann–Whitney U-test) was performed. In the case of qualitative variables, the comparison of proportions was tested by the Chi-square test or Fisher's exact test, whenever necessary. Also, odds ratios of COPD diagnosis in crude and multivariable analysis by logistic regression were estimated in the participants of the five areas of Spain that were assessed in all three studies. A p-value below 0.05 was considered to indicate statistical significance in all analyses. All statistics were performed using R (R Development Core Team, 2015).
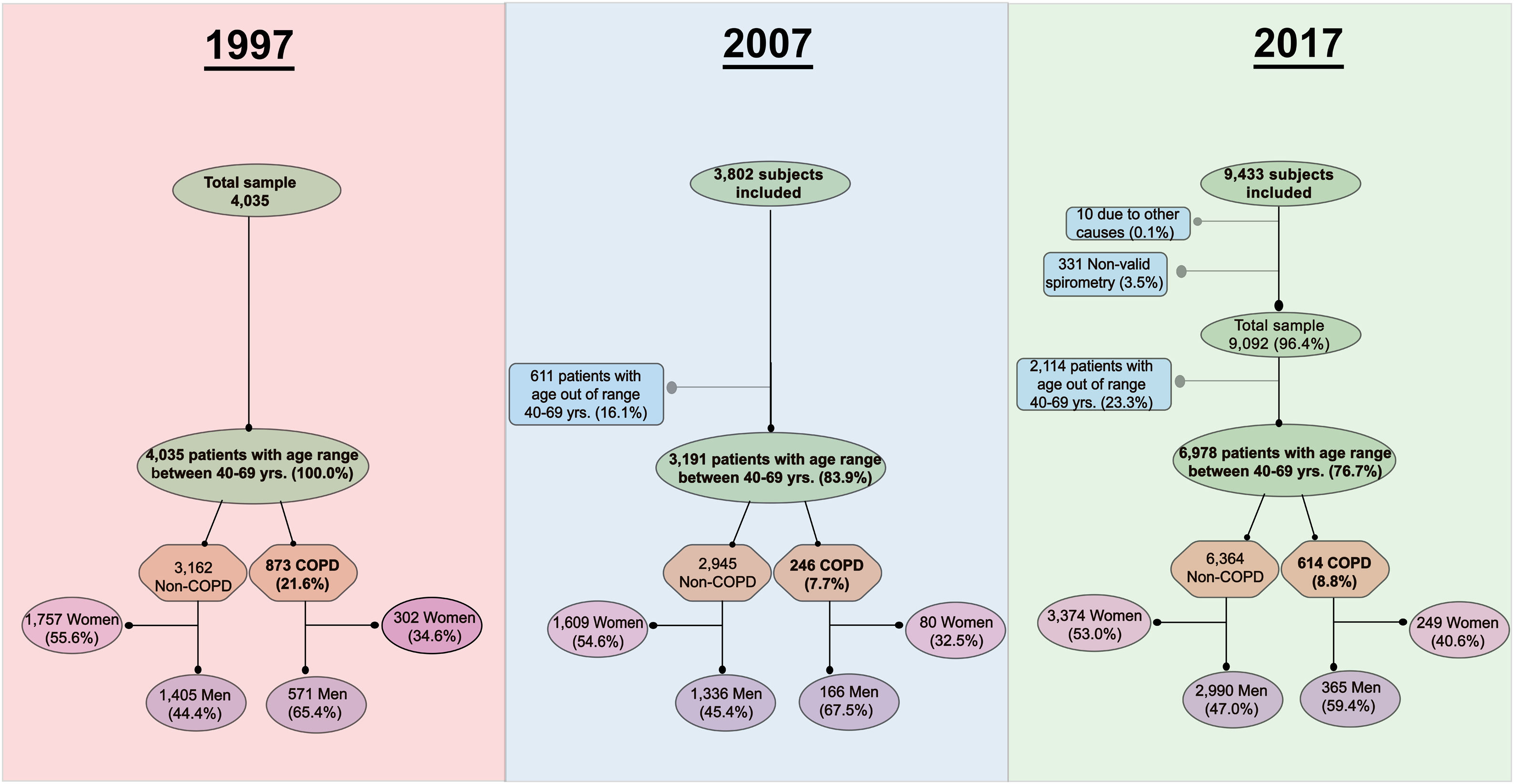
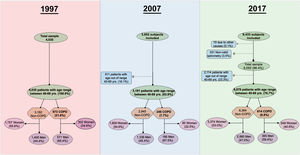
ResultsA flowchart with a detailed description of participation in all three studies is presented in Fig. 1. A total of 17,270 subjects agreed to participate, and a final group of 14,204 (82.2%) were available for analysis with valid spirometry.
The number of individuals who refused to participate was 1792 (30.8%) in IBERPOC, 389 (9.1%) in EPISCAN and 3392 (26%) in EPISCAN II. Regarding the characteristics of non participants, in EPISCAN they were slightly older and more frequently women and never and formed smokers; while in EPISCAN II the percentage of current smokers was similar, but the frequency of women and former smokers was higher in non participants.
Table 2 shows the demographic and clinical characteristics of the study population. The characteristics of the subgroups of 2007 and 2017 participants of age 40–69 years are presented as additional columns. When comparing IBERPOC participants vs EPISCAN and II subgroups, there were no statistically significant differences in gender, but in 2007 and 2017, participants were taller, with higher body mass index (BMI) and with a higher education, especially in 2017 (all p<0.001).
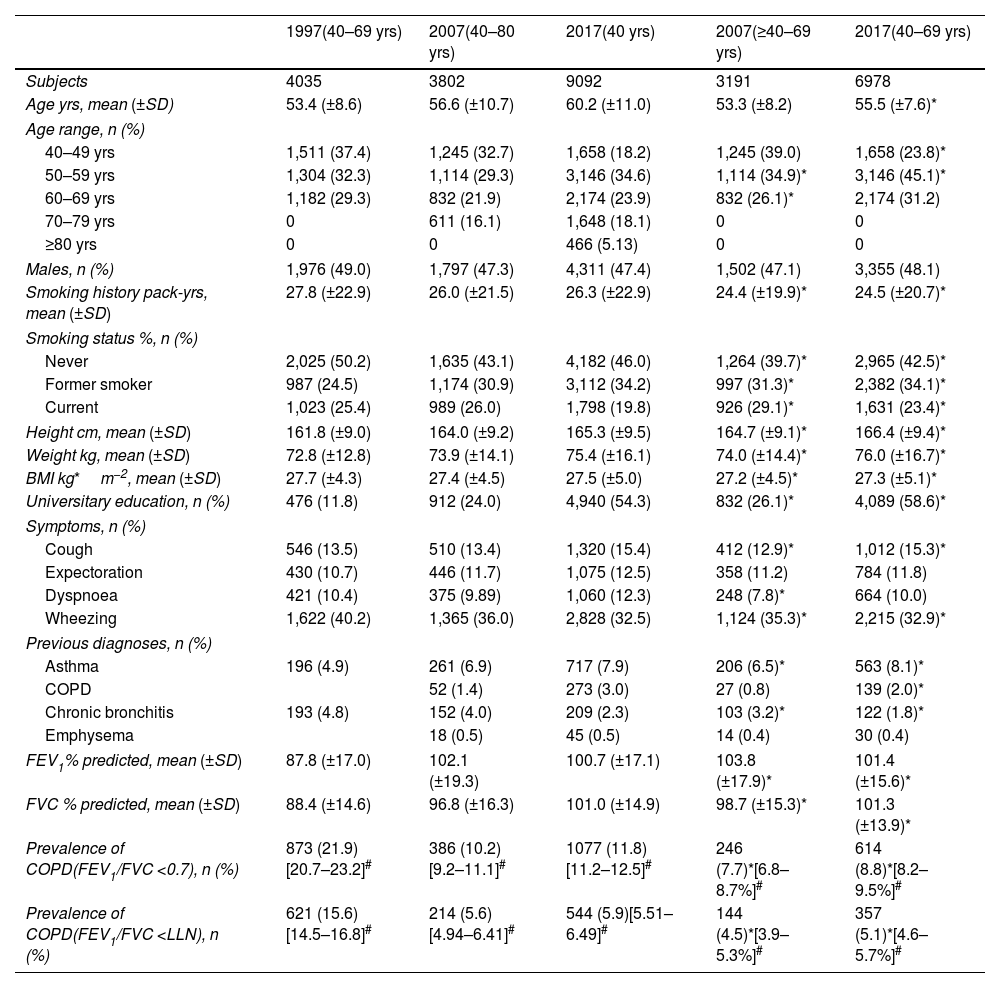
Demographic and Clinical Characteristics of Participants in 1997, 2007 and 2017, With All Age Ranges Available for Each Study.
| 1997(40–69 yrs) | 2007(40–80 yrs) | 2017(40 yrs) | 2007(≥40–69 yrs) | 2017(40–69 yrs) | |
|---|---|---|---|---|---|
| Subjects | 4035 | 3802 | 9092 | 3191 | 6978 |
| Age yrs, mean (±SD) | 53.4 (±8.6) | 56.6 (±10.7) | 60.2 (±11.0) | 53.3 (±8.2) | 55.5 (±7.6)* |
| Age range, n (%) | |||||
| 40–49 yrs | 1,511 (37.4) | 1,245 (32.7) | 1,658 (18.2) | 1,245 (39.0) | 1,658 (23.8)* |
| 50–59 yrs | 1,304 (32.3) | 1,114 (29.3) | 3,146 (34.6) | 1,114 (34.9)* | 3,146 (45.1)* |
| 60–69 yrs | 1,182 (29.3) | 832 (21.9) | 2,174 (23.9) | 832 (26.1)* | 2,174 (31.2) |
| 70–79 yrs | 0 | 611 (16.1) | 1,648 (18.1) | 0 | 0 |
| ≥80 yrs | 0 | 0 | 466 (5.13) | 0 | 0 |
| Males, n (%) | 1,976 (49.0) | 1,797 (47.3) | 4,311 (47.4) | 1,502 (47.1) | 3,355 (48.1) |
| Smoking history pack-yrs, mean (±SD) | 27.8 (±22.9) | 26.0 (±21.5) | 26.3 (±22.9) | 24.4 (±19.9)* | 24.5 (±20.7)* |
| Smoking status %, n (%) | |||||
| Never | 2,025 (50.2) | 1,635 (43.1) | 4,182 (46.0) | 1,264 (39.7)* | 2,965 (42.5)* |
| Former smoker | 987 (24.5) | 1,174 (30.9) | 3,112 (34.2) | 997 (31.3)* | 2,382 (34.1)* |
| Current | 1,023 (25.4) | 989 (26.0) | 1,798 (19.8) | 926 (29.1)* | 1,631 (23.4)* |
| Height cm, mean (±SD) | 161.8 (±9.0) | 164.0 (±9.2) | 165.3 (±9.5) | 164.7 (±9.1)* | 166.4 (±9.4)* |
| Weight kg, mean (±SD) | 72.8 (±12.8) | 73.9 (±14.1) | 75.4 (±16.1) | 74.0 (±14.4)* | 76.0 (±16.7)* |
| BMI kg*m−2, mean (±SD) | 27.7 (±4.3) | 27.4 (±4.5) | 27.5 (±5.0) | 27.2 (±4.5)* | 27.3 (±5.1)* |
| Universitary education, n (%) | 476 (11.8) | 912 (24.0) | 4,940 (54.3) | 832 (26.1)* | 4,089 (58.6)* |
| Symptoms, n (%) | |||||
| Cough | 546 (13.5) | 510 (13.4) | 1,320 (15.4) | 412 (12.9)* | 1,012 (15.3)* |
| Expectoration | 430 (10.7) | 446 (11.7) | 1,075 (12.5) | 358 (11.2) | 784 (11.8) |
| Dyspnoea | 421 (10.4) | 375 (9.89) | 1,060 (12.3) | 248 (7.8)* | 664 (10.0) |
| Wheezing | 1,622 (40.2) | 1,365 (36.0) | 2,828 (32.5) | 1,124 (35.3)* | 2,215 (32.9)* |
| Previous diagnoses, n (%) | |||||
| Asthma | 196 (4.9) | 261 (6.9) | 717 (7.9) | 206 (6.5)* | 563 (8.1)* |
| COPD | 52 (1.4) | 273 (3.0) | 27 (0.8) | 139 (2.0)* | |
| Chronic bronchitis | 193 (4.8) | 152 (4.0) | 209 (2.3) | 103 (3.2)* | 122 (1.8)* |
| Emphysema | 18 (0.5) | 45 (0.5) | 14 (0.4) | 30 (0.4) | |
| FEV1% predicted, mean (±SD) | 87.8 (±17.0) | 102.1 (±19.3) | 100.7 (±17.1) | 103.8 (±17.9)* | 101.4 (±15.6)* |
| FVC % predicted, mean (±SD) | 88.4 (±14.6) | 96.8 (±16.3) | 101.0 (±14.9) | 98.7 (±15.3)* | 101.3 (±13.9)* |
| Prevalence of COPD(FEV1/FVC <0.7), n (%) | 873 (21.9)[20.7–23.2]# | 386 (10.2)[9.2–11.1]# | 1077 (11.8)[11.2–12.5]# | 246 (7.7)*[6.8–8.7%]# | 614 (8.8)*[8.2–9.5%]# |
| Prevalence of COPD(FEV1/FVC <LLN), n (%) | 621 (15.6)[14.5–16.8]# | 214 (5.6)[4.94–6.41]# | 544 (5.9)[5.51–6.49]# | 144 (4.5)*[3.9–5.3%]# | 357 (5.1)*[4.6–5.7%]# |
BMI: body mass index; FEV1: forced expiratory volume in 1s; FVC: forced expiratory volume; % pred: % predicted; LLN: lower limit of normal.
Current and former smokers were more prevalent in 2007, with a significantly upward trend in smokers from 1997 to 2007 (25.4% vs 29.1%, p<0.001) and stability from 2007 to 2017 (23.4%).
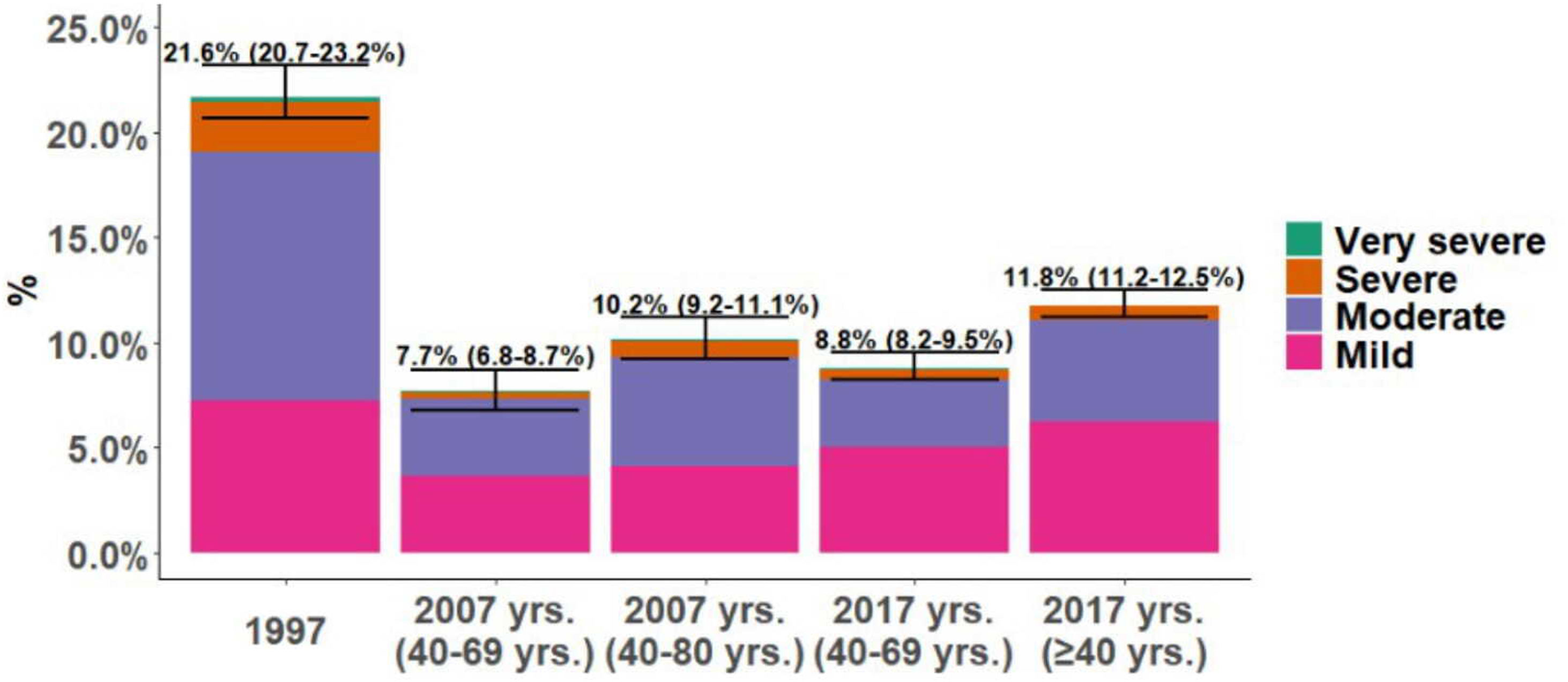
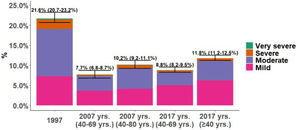
COPD PrevalenceThe prevalence of COPD defined by the GOLD criteria of those between 40 and 69 years decreased from 21.6% (95% CI 20.7%–23.2%) in 1997 to 8.8% (95% CI 8.2%–9.5%) in 2017, that is, a 59.2% decline (p<0.001). In 2007, the prevalence was 7.7% (95% CI 6.8%–8.7%) with a slight upward trend of 1.1 percentage points in 2017 (p=0.073). Prevalence by LLN criteria was lower than the fixed ratio in the three studies (Table 1). Trends were maintained when the analysis was performed using the LLN criteria and when all subjects were included (Fig. 2).
The distribution of COPD prevalence by severity according to GOLD classification changed with a reduction of all stages from 1997 to 2017 but in different proportions. In 1997, prevalence of mild COPD was 7.2%, moderate COPD 11.8% and 2.3% severe COPD, descending to 3.7%, 3.6%, 0.8%, respectively in 2007, and 5.0%, 3.2% and 0.4%, respectively in 2017. This trend was maintained in the analysis including all participants in 2007 and 2017 (additional columns in Fig. 2).
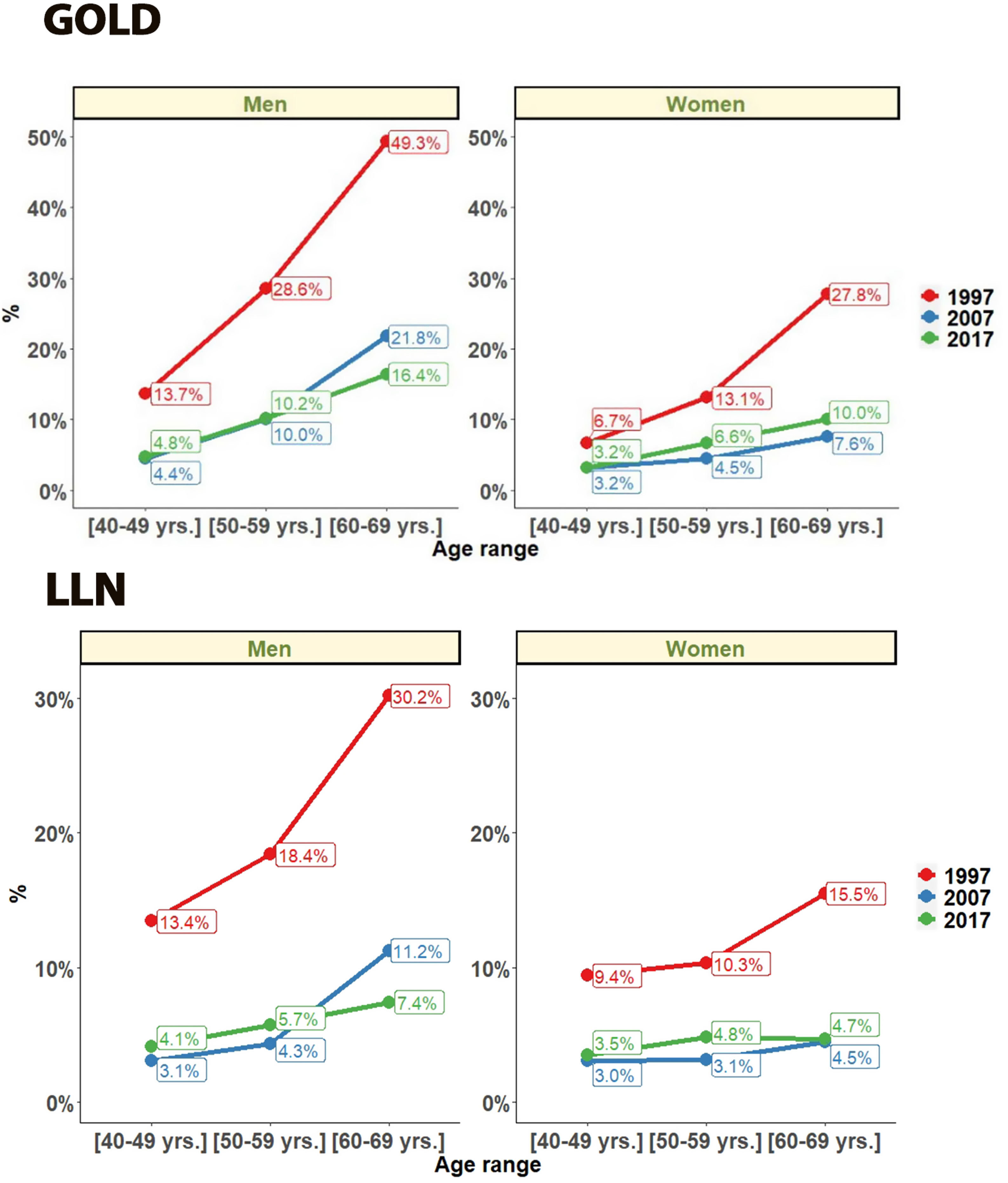
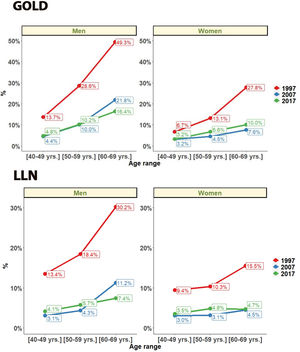
Regarding gender, COPD prevalence by GOLD criteria was higher in men than women. In men COPD prevalence decreased from 28.9% (95% CI 27.2%–31.2%) in 1997, to 15.1% (95% CI 13.5%–16.8%) in 2007 and 14.6% (95% CI 13.5%–15.6%) in 2017 (all p<0.001). In women COPD prevalence also decreased from 14.7% (95% CI 13.4%–16.5%) in 1997, to 5.7% (95% CI 4.7–6.7) in 2007 but with a significant increase to 9.4% (95% CI 8.5%–10.2%) in 2017 (all p<0.001). In all three studies, subjects aged >60 had a significantly higher prevalence (p<0.001) than younger participants (Fig. 3).
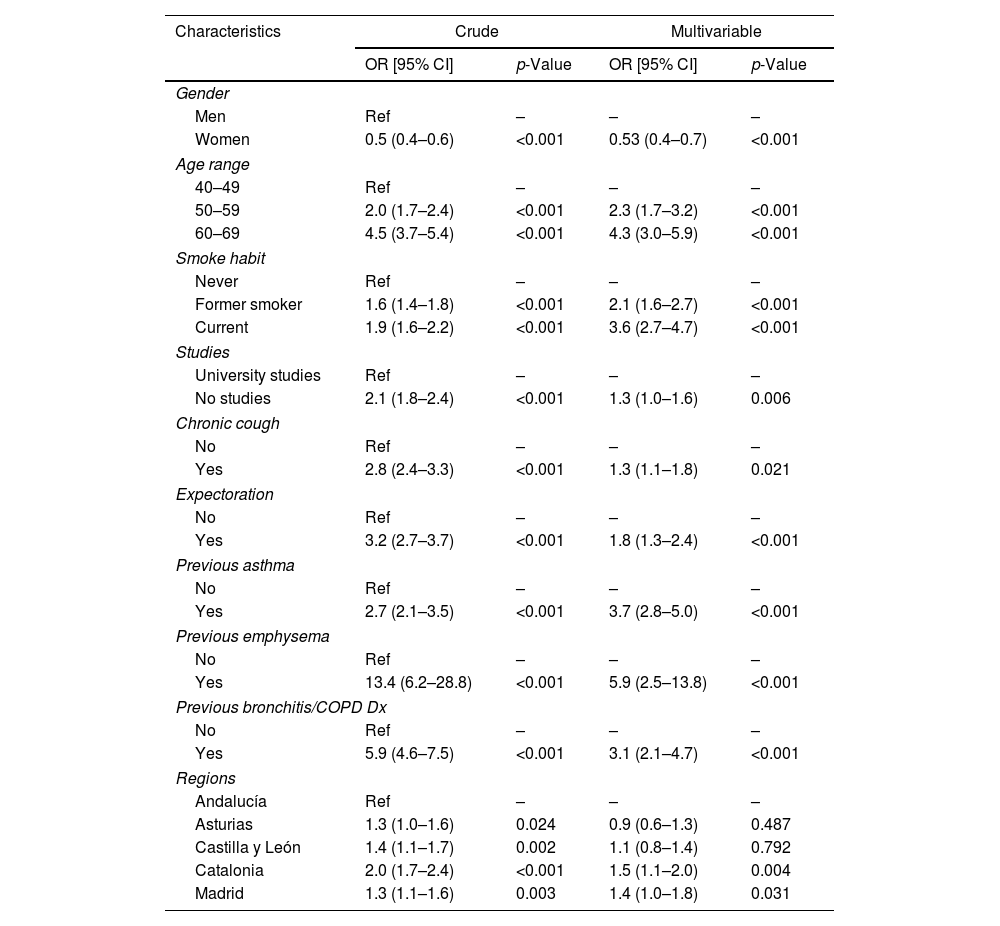
Factors Associated With COPDIn a multivariable analysis conducted in participants from the five regions in all three surveys, male gender, older age, smoking habit, lower education, previous diagnosis of bronchitis/COPD/asthma, emphysema and self-reported respiratory symptoms were positively associated with a higher probability of COPD (Table 3).
Odds Ratio for COPD Prevalence Assessed by GOLD, Crude and Multivariable.
| Characteristics | Crude | Multivariable | ||
|---|---|---|---|---|
| OR [95% CI] | p-Value | OR [95% CI] | p-Value | |
| Gender | ||||
| Men | Ref | – | – | – |
| Women | 0.5 (0.4–0.6) | <0.001 | 0.53 (0.4–0.7) | <0.001 |
| Age range | ||||
| 40–49 | Ref | – | – | – |
| 50–59 | 2.0 (1.7–2.4) | <0.001 | 2.3 (1.7–3.2) | <0.001 |
| 60–69 | 4.5 (3.7–5.4) | <0.001 | 4.3 (3.0–5.9) | <0.001 |
| Smoke habit | ||||
| Never | Ref | – | – | – |
| Former smoker | 1.6 (1.4–1.8) | <0.001 | 2.1 (1.6–2.7) | <0.001 |
| Current | 1.9 (1.6–2.2) | <0.001 | 3.6 (2.7–4.7) | <0.001 |
| Studies | ||||
| University studies | Ref | – | – | – |
| No studies | 2.1 (1.8–2.4) | <0.001 | 1.3 (1.0–1.6) | 0.006 |
| Chronic cough | ||||
| No | Ref | – | – | – |
| Yes | 2.8 (2.4–3.3) | <0.001 | 1.3 (1.1–1.8) | 0.021 |
| Expectoration | ||||
| No | Ref | – | – | – |
| Yes | 3.2 (2.7–3.7) | <0.001 | 1.8 (1.3–2.4) | <0.001 |
| Previous asthma | ||||
| No | Ref | – | – | – |
| Yes | 2.7 (2.1–3.5) | <0.001 | 3.7 (2.8–5.0) | <0.001 |
| Previous emphysema | ||||
| No | Ref | – | – | – |
| Yes | 13.4 (6.2–28.8) | <0.001 | 5.9 (2.5–13.8) | <0.001 |
| Previous bronchitis/COPD Dx | ||||
| No | Ref | – | – | – |
| Yes | 5.9 (4.6–7.5) | <0.001 | 3.1 (2.1–4.7) | <0.001 |
| Regions | ||||
| Andalucía | Ref | – | – | – |
| Asturias | 1.3 (1.0–1.6) | 0.024 | 0.9 (0.6–1.3) | 0.487 |
| Castilla y León | 1.4 (1.1–1.7) | 0.002 | 1.1 (0.8–1.4) | 0.792 |
| Catalonia | 2.0 (1.7–2.4) | <0.001 | 1.5 (1.1–2.0) | 0.004 |
| Madrid | 1.3 (1.1–1.6) | 0.003 | 1.4 (1.0–1.8) | 0.031 |
COPD: chronic obstructive pulmonary disease.
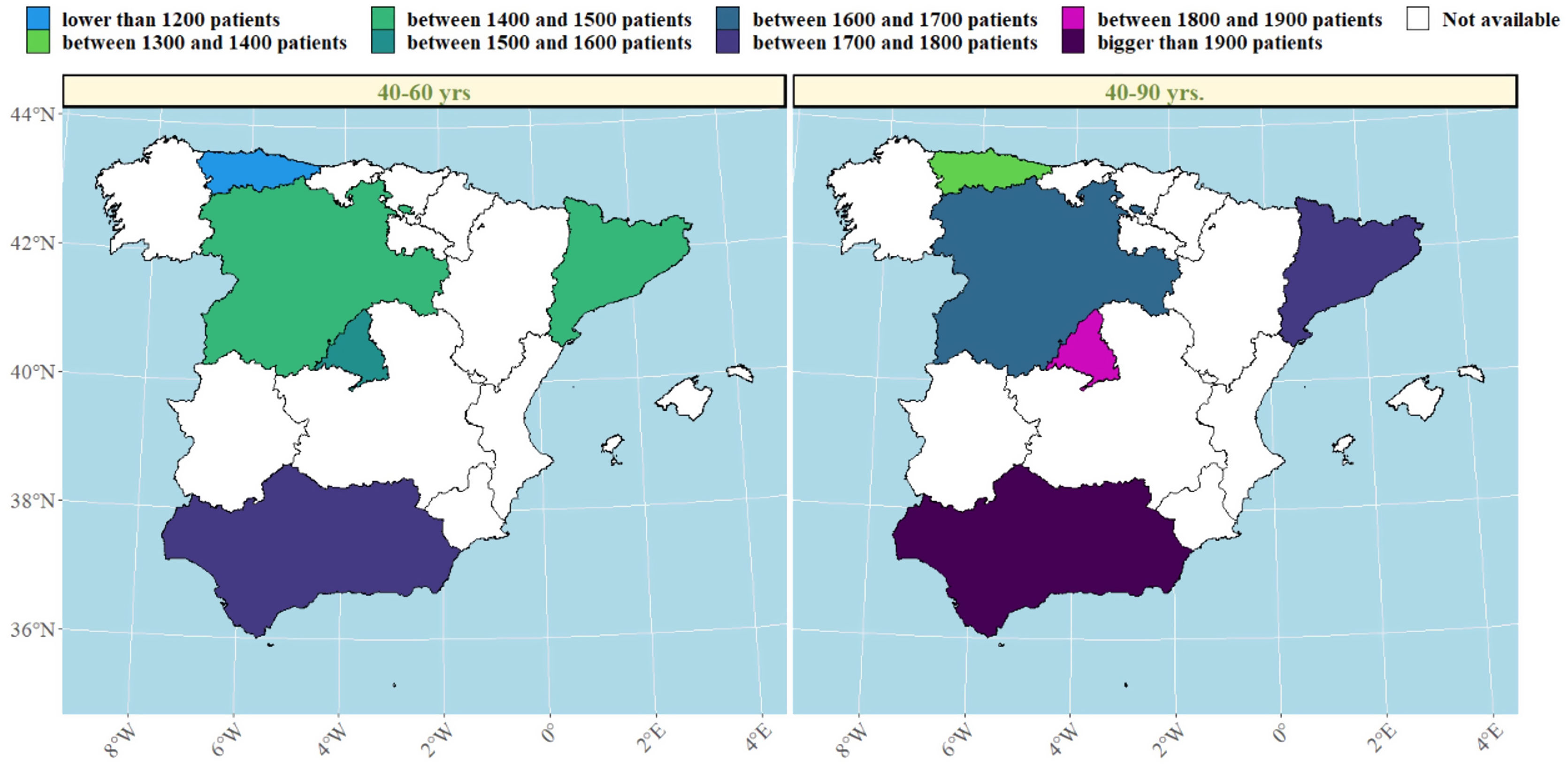
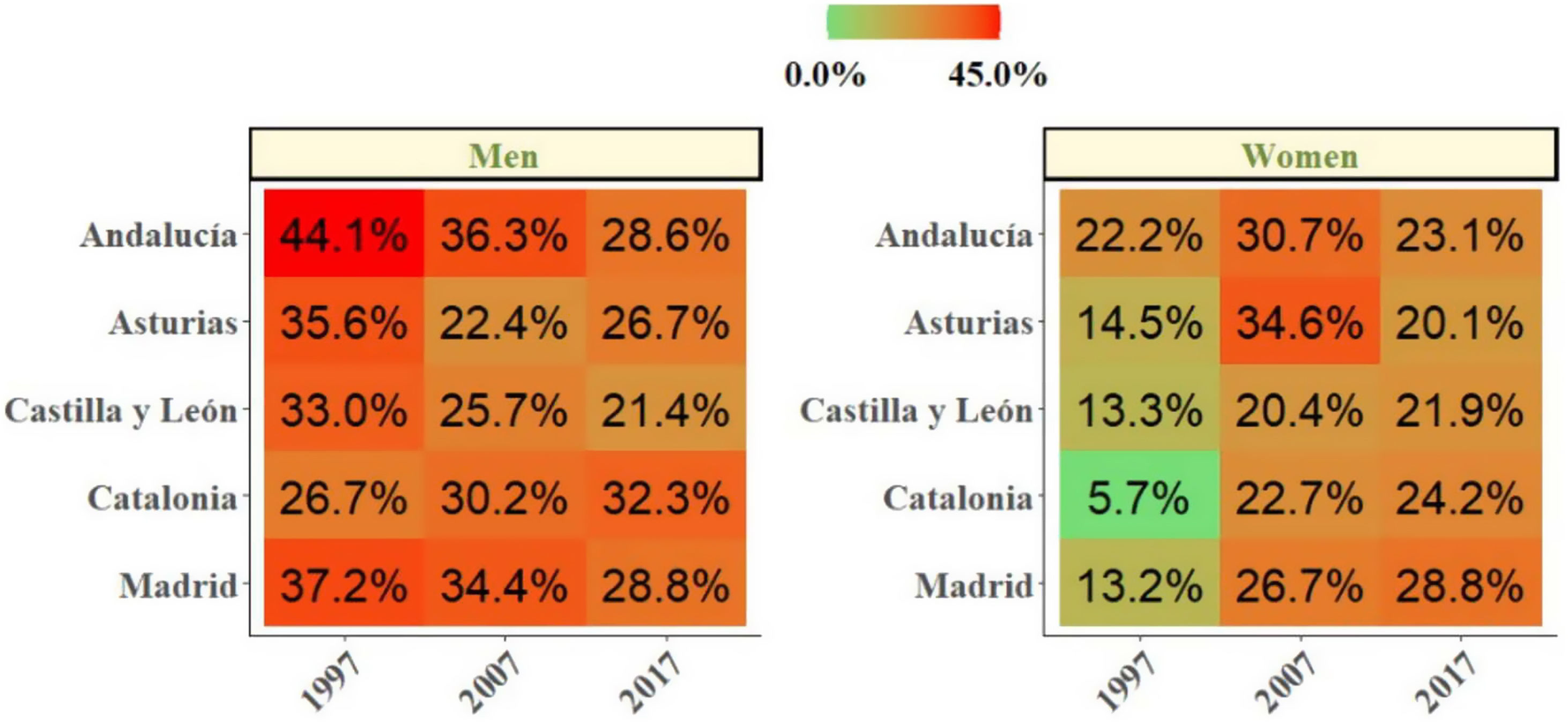
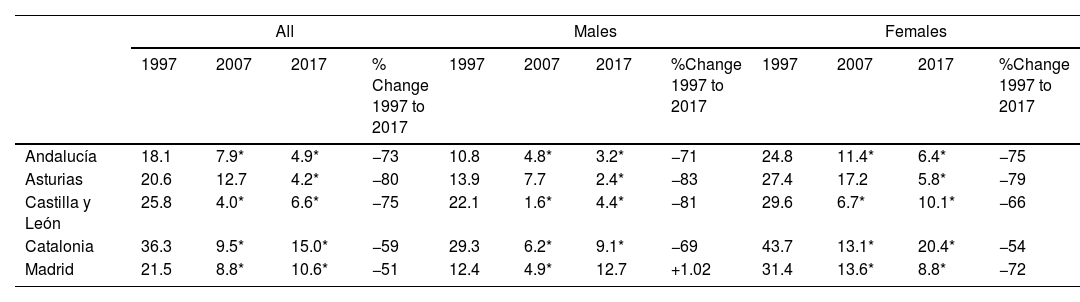
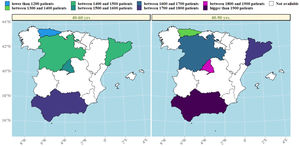
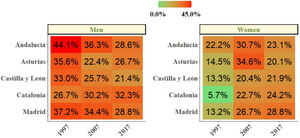
We then explored changes in COPD prevalence in the five areas of Spain (Oviedo, Vic-Manlleu-Barcelona, Burgos, Madrid and Sevilla) that were assessed in all three studies. These areas correspond to five different autonomous communities (Fig. 4).
There were large differences in the prevalence of COPD between regions within Spain.
The analysis of the studied areas showed a substantial decrease in local COPD prevalence, but differences among the five regions were also observed. The highest prevalence of COPD was observed in Catalonia in 1997, which was over two-fold that was observed in Andalucía, the lowest one. These differences were maintained in 1997 and 2017 (Table 4). In Madrid, Catalonia and Castilla-León, a slightly upward trend was found between 2007 and 2017. There were also substantial differences found in smoking habit. In 1997, the lowest smoking prevalence was found in Catalonia, with 15.9% of current smokers. However, in 2017, Catalonia was one of the areas with the highest smoking rate (28.4%). On the contrary, in 1997, Andalucía was the region with the highest percentage of smokers (33.7%) with an important smoking reduction in 2017 (26%). Asturias and Castilla-León showed a homogeneous trend throughout the three studies with low smoking rates (Table 1 supplementary). In men, smoking habit decreased in all regions, except in Catalonia, where an upward trend was observed in the last decades. In women, on the contrary, an increase in smokers was observed in all regions, especially in Catalonia (Fig. 5).
Changes Between 1997 and 2017 COPD Prevalence in the Five Repeater Areas, Total and by Sex in 40–69 years old.
| All | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | 2007 | 2017 | % Change 1997 to 2017 | 1997 | 2007 | 2017 | %Change 1997 to 2017 | 1997 | 2007 | 2017 | %Change 1997 to 2017 | |
| Andalucía | 18.1 | 7.9* | 4.9* | −73 | 10.8 | 4.8* | 3.2* | −71 | 24.8 | 11.4* | 6.4* | −75 |
| Asturias | 20.6 | 12.7 | 4.2* | −80 | 13.9 | 7.7 | 2.4* | −83 | 27.4 | 17.2 | 5.8* | −79 |
| Castilla y León | 25.8 | 4.0* | 6.6* | −75 | 22.1 | 1.6* | 4.4* | −81 | 29.6 | 6.7* | 10.1* | −66 |
| Catalonia | 36.3 | 9.5* | 15.0* | −59 | 29.3 | 6.2* | 9.1* | −69 | 43.7 | 13.1* | 20.4* | −54 |
| Madrid | 21.5 | 8.8* | 10.6* | −51 | 12.4 | 4.9* | 12.7 | +1.02 | 31.4 | 13.6* | 8.8* | −72 |
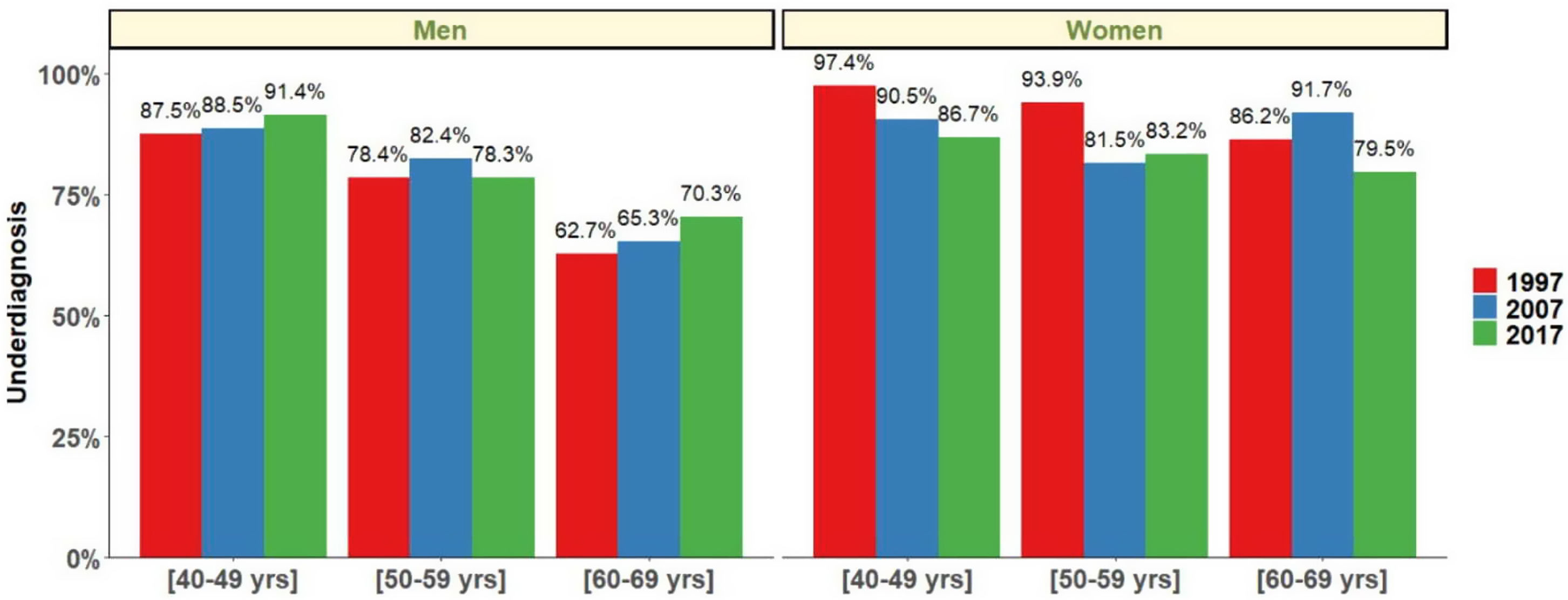
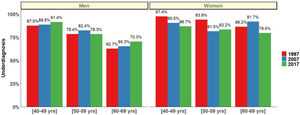
Finally, COPD underdiagnosis showed no statistically significant differences between 1997, 2007 and 2017 in participants 40–69 years. In 1997 COPD underdiagnosis was 77.6.% (95% CI 72.9–82.9), 78.4% (95% CI 74.8–81.2) in 2007 and 78.2% in 2017 (95% CI 73.5–81.1) (p=0.95). Undiagnosed COPD was higher in the younger ages (40–49 yrs and 50–59 yrs) and was higher in women than in men in all three studies (p<0.05) (Fig. 6).
DiscussionThe results of this analysis conclude that prevalence of GOLD-defined COPD in Spain, in the population aged 40–69 years between 1997 and 2017, has experienced a substantial decrease of 59.2%. Nevertheless, in the last decade there are no relevant changes (from 7.7% to 8.8%), with a virtual plateau. Prevalence was higher in men than in women, and differences in trends by gender were observed. Prevalence in men decreased in the three studies sequentially, while among women the trend has been the opposite, with a significant increase in prevalence in the last ten-year period. Age, cumulative tobacco consumption, low education level, previous diagnosis of bronchitis/COPD and self-reported respiratory symptoms were the strongest risk factors for the disease.
Publications on COPD prevalence have not been very common until 2001, with only 32 epidemiology studies published to date, with prevalence ranging from 0.23 to 18.3% and different definitions based on spirometry, respiratory symptoms and patient-reported disease.21 In our study, disease prevalence has been defined on a postbronchodilator spirometric criteria in order to draw comparisons with most epidemiological studies published to date.3,4,22 Although full clinical assessment of symptoms, signs and risk factors is indeed fundamental to diagnose and stage COPD at the patient level, all epidemiological studies, from the CIBA Symposium in 1958 to date, only use spirometry to determine COPD at the population level. Main reason being there are no universally agreed standards on questionnaires and thresholds of neither symptoms, signs nor risk factors for their assessment at the population level (actually, same applies to spirometry). So, we have to abide that any spirometry-only assessment of COPD is a limitation in the absence of any population consensus.
The decrease in COPD prevalence in the present study in Spain during the last two decades follows other European studies and one study from NHANES, that reported a slight decrease in prevalence.23–25 Likewise, the Global Burden of Disease, reported in high-income super-region, an increase of 1.5 percentage points from the prevalence in 1990 (4.4%) to the prevalence in 2017 (5.9%).1 In our study, this upward trend was observed between 2007 and 2017. However, in a study from Finland, no change in prevalence was found, and in a recent meta-analysis, an increase in COPD prevalence from the European region of 22.5% was reported from 1990 to 2010.26,27 These different results, highlight the difficulty in comparing population findings of forced spirometry because the studies are conducted in different countries and geographic areas with different machines and protocols, in populations with varying smoking habits and exposure to environmental risk factors. In 1997, COPD prevalence was higher than in later studies with a great descent in the first decade and stability in the last one.3 Technical changes in spirometry may have influenced our results; In IBERPOC it was a turbine spirometer, while in EPI-SCAN a pneumotacograph spirometer with high sensitivity was used. It has been reported that turbine spirometers create greater internal resistance to flow. An increase in internal resistance of these spirometers can produce an underestimation of expiratory volumes at low flow, detecting less COPD, which could have occurred in the IBERPOC study. As this effect occurs lastly in the expiratory manoeuvre, since most resistance occurs at low flows, it appears that the measure should affect FVC more than the FEV1 and, consequently, an increase of internal resistance of the spirometer could result in an overestimation of the FEV1/FVC ratio.28 Also, methodological differences in the studies like the sampling frame and recruitment with the possibility of selection of two different populations in IBERPOC and in EPISCAN studies, may have influenced the results. The cohort of IBERPOC in 1997 suffered the consequences of the Spanish Civil War, from 1936 to 1939 with extremely hard living conditions, malnutrition and more childhood infections, like tuberculosis, that might have influenced lung development.29 As a result of this, IBERPOC participants could not attain normal maximal lung function by early adulthood with a different trajectory suggesting that genetic risk, prenatal and early life factors, and exposures in adulthood contribute to COPD pathogenesis in each individual.30
It is well known that prevalence may vary according to the spirometric criteria used and the preferred criterion for diagnosing airway obstruction in COPD remains controversial. Current guidelines continue to recommend using a fixed post-bronchodilator ratio.20,31 However, some studies have found that the use of a fixed FEV1/FVC ratio will result in underestimation of COPD in younger individuals (particularly those with mild disease) and may overestimate the prevalence of COPD in older adults, proposing the use of LLN as a more specific tool to diagnose airflow obstruction.32 Our study highlights these known differences with higher percentages obtained according to fixed FEV1/FVC ratio among older participants. In contrast, no differences between LLN and fixed post-bronchodilator ratio in younger participants (40–50 years) were found. In our study, COPD severity mostly was in mild stages, so we believe that the use of the fixed ratio is more appropriate in population studies since the use of LLN criterion (usually more restrictive) may delay diagnosis and treatment.33
Our results suggest that the gender gap in COPD is narrowing. Similar to previous studies, the prevalence of COPD was consistently higher among men but has risen more rapidly in women than men, equaling that of men since 2008.26,27,34 In the present study, overall fixed-ratio COPD in men decreased, and the decrease was across all age groups; however, in women in the last two decades, an increase in prevalence was observed in females aged ≥50 years. This upward trend of COPD prevalence in women is closely related to the pattern of smoking, ageing and urbanized regions settings. In Spain, the percentage of daily smokers decreased from 1980 to 2016, with a higher decrease in men (from 41 to 26%) than in women (from 21 to 17%), in whom there is an apparent plateau from 2005.35 In our study, in 2017, the highest smoking prevalence were observed in Madrid and Catalonia (Barcelona), probably influenced by higher socioeconomic status and urban settings.36 It is established that women appear to be more susceptible to the effects of cigarette smoke than men. Hence, our study confirms that women are a vulnerable group and thus are an essential target population for tobacco control interventions aimed at decreasing nicotine addiction initiation.
Lamprecht et al.37 in an analysis of four epidemiologic surveys, described COPD underdiagnosis was more frequent in men than in women worldwide, except in Spain with higher underdiagnosis in women. This difference in gender in Spain might be due to different causes.38 First of all, physicians might be less likely to consider COPD when confronted by a female patient with respiratory symptoms because it is still a more prevalent disease in men. Anxiety and depression sometimes lead to a different perception of dyspnoea among women not associating these symptoms to disturbed lungs. Further, the under-utilization of spirometry and the increase of smoking in young women and adolescents can explain underdiagnosis in our population.39,40
Our study has several strengths. It is one of the largest national population studies published with a long time window of 20 years, over which trends were studied. Given this large sample size and with a high response rate, it can be considered that the final sample of participants is representative of the Spanish population between 40 to 69 years. Finally, high-quality spirometry and post-bronchodilator testing adhered to the strictest international guidance and protocols, reducing bias due to methodological issues, especially between 2007 and 2017.
Assessment of temporal trends in the prevalence of COPD is complex, so some limitations must also be discussed. Diagnosis is based solely on spirometry (airflow limitation), consistent with other epidemiological studies on COPD internationally,3 but this may include other obstructive diseases like asthma. The difference between spirometers may affect the estimation of FEV1/FVC with higher COPD prevalence detected on 1997.28 Roca et al. equations were used in 1997 with predicted values obtained in the mid-1980s.14 Later, the average Spanish population has grown taller 30 years later, so the comparison with Quanjer et al. values used in 2007 and 2017 could not be quite appropriate.16 Most areas surveyed were urban, so rural areas are under-represented. Choosing major cities does not in itself constitute a selection bias, but was instead a reasoned, practical, executive, methodological decision, since the practical aspects of inviting people who live in urban capital optimises participation, where research teams and certified lung function laboratories are concentrated; Finally, neonatal, childhood risk factors, occupational and environmental exposures were not analyzed in all the studies, with a possible influence in the results.
From a Public Health perspective, a large reduction in COPD prevalence, while a sustained high underdiagnosis of the same condition is observed, might seem counterintuitive. The former is positive news, while the later is not. However, as the natural history of COPD and airflow limitation have yet to be unraveled by life-long cohort studies with lung function from birth to old ages, COPD might still deliver surprising results.
ConclusionIn conclusion, we report a substantial reduction of 59.2% in the prevalence of COPD in Spain from 1997 to 2017 in subjects aged 40–69 years, with no relevant changes between 2007 and 2017. This study highlights the increasing prevalence of COPD in the female population and higher underdiagnosis in women than men, and in young people. It is necessary to identify local factors that can affect this trends and act accordingly with more strategies to reduce the very high COPD underdiagnosis.
Ethical ApprovalThe three studies were approved by the Ethics Committees of the participating hospitals and all subjects provided written informed consent to participate in the studies.
FundingIBERPOC study funding was obtained from Boehringer Ingelheim, Spain, S.A. EPISCAN study was a GSK sponsored GlaxoSmithKline Spain. EPISCAN II study was a GSK sponsored study, registered in ClinicalTrials.gov Identifier: NCT01122758.
Conflict of InterestThe authors declare that they have no real or perceived conflicts of interest with the submitted manuscript.
Elena García Castillo has received speaker or consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline and Bial. Tamara Alonso Pérez has received speaker or consulting fees from AstraZeneca, Chiesi and GlaxoSmithKline. Julio Ancochea has received company training fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Novartis and speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Roche and Faes Farma.
Borja G. Cosio has received speaker or consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Sanofi, TEVA, and research grants from Menarini, AstraZeneca and Boehringer-Ingelheim. Francisco García-Río has received speaker or consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Pfizer and Rovi, and research grants from Chiesi, Esteve, Gebro Pharma, GlaxoSmithKline, Menarini and TEVA. Juan José Soler-Cataluña has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline, Menarini, Novartis and Teva, consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, GlaxoSmithKline, Ferrer and Novartis, and research grants from GlaxoSmithKline. Guadalupe Sánchez-Herrero is a Medical Department GSK employee. All others authors declare no conflicts of interest.
We sincerely thank the scientific committee of IBERPOC: Sobradillo V, Jiménez CA, Gabriel R, Viejo JL, Masa JF, Fernández-Fau L, Villasante C.