Among patients hospitalized for an exacerbation of chronic obstructive pulmonary disease (COPD), the SLICE trial showed that the addition of an active diagnostic strategy for pulmonary embolism (PE) to usual care compared with usual care alone did not improve a composite set of health outcomes. The objective of this subanalysis was to determine the frequency and prognostic significance of findings on computed tomography pulmonary angiogram (CTPA) supporting an alternative diagnosis to PE.
MethodsWe analyzed all patients randomized to the intervention in the SLICE trial who received a CTPA that did not show PE. We used multivariable logistic regression to assess the independent association between findings supporting an alternative diagnosis to PE and a composite of readmission for COPD or death within 90 days after randomization.
ResultsAmong the 746 patients who were randomized, this subanalysis included 175 patients in the intervention group who received a CTPA that did not show PE. Eighty-four (48.0%) patients had acute bronchial infection, 13 (7.4%) had lung cancer, 10 (5.7%) had congestive heart failure, 8 (4.6%), 18 (10.3%) had other diagnoses, and 42 (24.0%) had a normal CTPA. In multivariable analysis, findings supporting an alternative diagnosis to PE were not significantly associated with the primary outcome (odds ratio: 0.64; 95% confidence interval: 0.30–1.38; P=0.26).
ConclusionsAmong patients hospitalized for an exacerbation of COPD, CTPA identified an alternative diagnosis in 76% of the patients. However, specific management of these patients was not associated with improved outcomes within 90 days after randomization.
El ensayo clínico SLICE no demostró un beneficio clínico de la búsqueda activa de la tromboembolia de pulmón (TEP) en pacientes que requirieron ingreso por agudización de su enfermedad pulmonar obstructiva crónica (EPOC). El objetivo de este subanálisis fue determinar la frecuencia y el significado pronóstico del hallazgo de un diagnóstico alternativo a la TEP en la tomografía computarizada de tórax (TC) realizada a pacientes hospitalizados por EPOC agudizada.
MétodosAnalizamos los pacientes del grupo intervención de SLICE que recibieron una TC con resultado negativo para TEP. Evaluamos mediante el uso de regresión logística multivariable la asociación entre un diagnóstico alternativo y el evento primario compuesto de reingreso por EPOC y la mortalidad por cualquier causa en los primeros 90 días después de la aleatorización.
ResultadosEste análisis incluyó a 175 pacientes del grupo intervención que recibieron una TC con un resultado negativo para TEP. De ellos, 84 (48,0%) fueron diagnosticados de infección bronquial aguda, 13 (7,4%) de cáncer de pulmón, 10 (5,7%) de insuficiencia cardiaca, 8 (4,6%) de neumonía, 18 (10,3%) de otros diagnósticos, y en 42 (24,0%) pacientes la TC fue normal. En el análisis multivariable, un diagnóstico alternativo no se asoció de manera significativa con el evento primario (odds ratio: 0,64; IC 95%: 0,30-1,38; p=0,26).
ConclusionesEntre los pacientes que requieren ingreso por agudización de EPOC, la TC ofrece un diagnóstico alternativo a la TEP en el 76% de las ocasiones. El tratamiento específico de estas patologías no se asocia a un mejor pronóstico durante el seguimiento.
Chronic obstructive pulmonary disease (COPD) is a common cause of morbidity and mortality worldwide.1,2 During the course of their disease, patients with COPD may experience exacerbations (defined as acute worsening of respiratory symptoms requiring intensified treatment) that can lead to hospitalization, increase the risk of further exacerbations, and worsen their prognosis.3,4
Some diseases such as heart failure, pneumothorax or pulmonary embolism (PE) can mimic (or coexist with) a COPD exacerbation.5 The results of studies investigating the prevalence of PE in this group of patients have been inconsistent.6–8 A French study that included 211 patients with COPD exacerbations of unknown cause found a prevalence of PE of 21.8%, 95% of which were central or segmental.6 However, Couturaud et al. reported a prevalence of PE of 5.9% in a series of 740 patients with COPD exacerbation requiring hospital admission.9 Recently, the SLICE (Significance of Pulmonary Embolism in COPD Exacerbations) multicenter clinical trial evaluated the efficacy and safety of an active strategy for diagnosing PE in patients requiring hospital admission due to exacerbation of their COPD.10 In this trial, proactive screening for PE had no statistically significant effect on improving the primary composite endpoint of venous thromboembolism (VTE), readmission for COPD, or death from any cause within 3 months of randomization.11
Although the results of the SLICE trial showed that proactive diagnosis of PE in these patients has no clinical benefit, some studies suggest that chest computed tomography (CT) is more sensitive than chest radiography for the diagnosis of comorbidities that mimic COPD exacerbation,12 and clinical practice guidelines recommend identifying the precipitating factor in order to administer appropriate treatment.5 Nevertheless, there is no evidence that identifying a comorbidity on chest CT and administering targeted treatment will improve prognosis in these patients.
The aim of this subgroup analysis was to calculate the number of patients hospitalized for COPD exacerbations in whom chest CT provided an alternative diagnosis to PE, and to determine the prognostic significance of diagnosing and specifically treating these comorbidities.
MethodStudy design and populationThis is a subgroup analysis of the SLICE multicenter clinical trial. The protocol and main analysis of SLICE have been published elsewhere.10,11 In brief, SLICE randomized consecutive patients requiring hospitalization for exacerbation of their COPD to proactive screening for PE or conventional management based on local protocols and clinical practice guidelines. Screening for PE initially consisted of performing a D-dimer test. A negative D-dimer (according to the cut-off point used in each participating center) excluded PE. Patients with a positive D-dimer test underwent chest CT angiography within 12h of randomization.10 A negative CT angiography ruled out PE, while a positive CT angiography was an indication for anticoagulant treatment.
In this subgroup analysis, we included all patients in the intervention group of the SLICE trial that tested negative for PE on chest CT angiography (i.e., normal CT or CT finding of an alternative diagnosis to PE).
Variables analyzed and definition of alternative diagnosesDemographic, clinical, and laboratory data were collected at the time of randomization. These included, among others: age, sex, weight, height, comorbidities (cancer [non-lung], ischemic heart disease, heart failure, sleep apnea), risk of VTE (previous surgery, immobilization, history of VTE), severity of COPD, number of exacerbations in the preceding year, treatment for the stable phase of COPD, clinical signs and symptoms (heart rate, blood pressure, blood oxygen) and laboratory test results at the time of admission (hemoglobin, leukocytes, platelets, serum creatinine).
Bronchial infection on chest CT angiography was defined as bronchial wall thickening, centrilobular nodules, and a tree-in-bud sign. Pneumonia was diagnosed in patients with a lobar or segmental alveolar pattern who showed good clinical and radiological response to broad-spectrum antibiotic therapy. Heart failure was diagnosed in patients with interstitial involvement (Kerley lines), an alveolar or ground-glass pattern predominantly in dependent regions, and bilateral pleural effusion. Histological confirmation of pulmonary nodules and masses was required for a diagnosis of cancer.
Episodes analyzedThe primary outcome variable was the composite of readmission for COPD or all-cause mortality at 90 days. Secondary variables were each of the individual components of the primary variable, new onset or recurrent of non-fatal thrombotic events, and length of hospital stay.
Statistical analysisContinuous data are shown as mean±standard deviation or median (interquartile range), and categorical data are shown as absolute values and proportions (%). Differences between groups were tested using the Student's t-test or the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher's exact test for categorical variables.
A multivariate logistic regression model was constructed to determine whether an alternative diagnosis on CT (versus normal CT) was independently correlated with the primary outcome variable.13 The model was constructed using a maximum of 10 variables that showed a univariate correlation with the outcome (P<0.05) or that were considered clinically relevant based on expert opinion and published experience.14 These variables were: (a) age; (b) sex; (c) COPD severity; (e) congestive heart failure (CHF); (f) cancer; (g) an alternative diagnosis on CT; (h) immobilization; (i) heart rate; (j) systolic blood pressure; and (k) blood oxygen levels. Starting with the full model, we used the backward elimination technique to eliminate any variables that did not modify the coefficient of the variable (i.e., presence of an alternative diagnosis on CT) by 10% or more. Odds ratios (OR) were calculated with their 95% confidence intervals (CI). We then repeated the analyses to assess the prognostic significance of the presence (versus absence) of any diagnosis, including PE, on chest CT. As the model was not adjusted for multiplicity, the analyses of the secondary variables should be viewed as merely exploratory.
A two-sided P<0.05 was considered indicative of statistical significance in all cases. All analyses were performed on Stata, version 13.1 (StataCorp LLC; College Station, Texas, US).
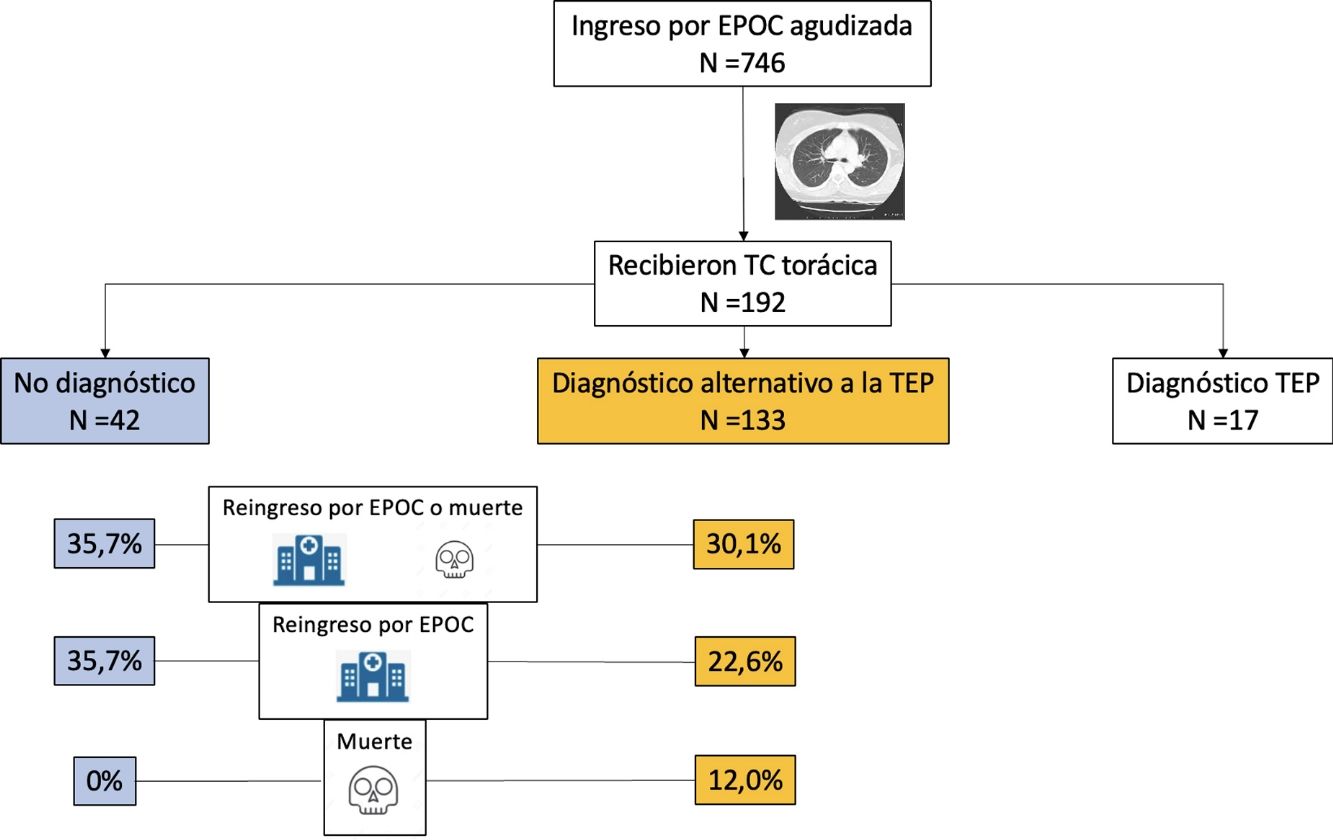
ResultsWe evaluated 1003 patients between September 2014 and July 2020; 746 (74.4%) of these were randomized. Of the 369 patients in the intervention group with a positive D-dimer test, 192 underwent chest CT, which was positive for PE in 17 (8.9%) cases. We also analyzed data from the 175 patients in the intervention group that tested negative for PE on chest CT angiography (i.e., normal CT or CT finding of an alternative diagnosis to PE). Mean age was 72.9 (9.7) years and 78.9% were men.
Chest CT diagnoses and changes in treatmentChest CT findings were suggestive of an alternative diagnosis to PE in 133 patients (76.0%; 95% CI, 69.0–82.1%). Of these, 84 (48.0%) were diagnosed with acute bronchial infection, 13 (7.4%) with lung cancer, 10 (5.7%) with heart failure, 8 (4.6%) with pneumonia, 18 (10.3%) with other diagnoses (5 with bronchiectasis, 4 with fibrosis, 3 with pleural effusion, 2 with pulmonary hypertension, 2 with pericardial effusion, 1 with hypersensitivity pneumonitis, and 1 with organizing pneumonia). Chest CT was normal in 42 (24.0%) patients. Diagnosis of these comorbidities led to a change in the treatment of 31 patients (31 of 133 patients; 23.3%; 95% CI, 16.4–31.4%): diuretics in 10 patients, antibiotics in 9, systemic corticosteroids in 7, and treatment for lung cancer in 5 patients.
Demographic, clinical and laboratory characteristics are shown in Table 1. There were no significant differences between the group of patients with an alternative diagnosis to PE on CT and the group of patients with normal CT. Purulent sputum during exacerbation was found more frequently in patients with an alternative diagnosis to PE on CT (8.3% versus 0%; P=0.07).
Main characteristics of the 175 patients.
| No. (%) of patients | |||
|---|---|---|---|
| With alternative diagnosis on CT(N=133) | With normal CT(N=42) | P value | |
| Age>75 years | 64 (48.1) | 19 (45.2) | 0.86 |
| Sex | |||
| Male | 107 (80.5%) | 31 (73.8%) | 0.39 |
| Female | 26 (19.5%) | 11 (26.2%) | – |
| Active smoker | 38 (28.6%) | 10 (23.8%) | 0.69 |
| Pack-years, mean (SD), no. | 55.7 (20.8) | 71.2 (35.4) | 0.21 |
| COPD exacerbations in the previous 12 months, mean (SD), no. | 1.3 (1.7) | 1.7 (2.0) | 0.21 |
| Post-bronchodilator FEV1, mean (SD), % predicted | 48.4 (18.6) | 47.2 (20.7) | 0.75 |
| Very severe COPD: <30% predicted | 13 (9.8) | 8 (19.0) | 0.42 |
| Severe COPD: 30% to <50% predicted | 62 (46.6) | 17 (40.5) | – |
| Moderate COPD: 50% to <80% predicted | 45 (33.8) | 14 (33.3) | – |
| Mild COPD: ≥80% predicted | 13 (9.8) | 3 (7.1) | – |
| VTE risk factors | |||
| Immobilizationa | 33 (24.8) | 6 (14.3) | 0.20 |
| Sleep apnea | 17 (12.8) | 8 (19.0) | 0.32 |
| Heart failure | 27 (20.3) | 5 (11.9) | 0.26 |
| Cancerb | 8 (6.0) | 1 (2.4) | 0.69 |
| History of VTE | 3 (2.3) | 1 (2.4) | 1.0 |
| Surgeryc | 0 (0) | 0 (0) | – |
| Symptoms and signs | |||
| Dyspnea | 132 (99.2) | 42 (100) | 0.75 |
| Heart rate>100/min | 40 (30.1) | 16 (38.1) | 0.35 |
| Increased sputum volume | 46 (34.6) | 13 (31.0) | 0.71 |
| Purulent sputum | 11 (8.3) | 0 (0) | 0.07 |
| Systolic blood pressure<100mmHg | 6 (4.5) | 1 (2.4) | 1.0 |
| SatO2<90% | 58 (43.6) | 14 (33.3) | 0.28 |
| Clinical probability (Wells scale) | |||
| Low | 56 (17.0) | 20 (47.6) | 0.59 |
| Intermediate | 77 (83.0) | 22 (52.4) | – |
| High | 0 (0) | 0 (0) | – |
| Lab tests | |||
| Creatinine, mean (SD), mg/dL | 0.9 (0.3) | 0.9 (0.2) | 0.71 |
| Creatinine>1.5g/dL | 4 (3.0) | 0 (0) | 0.57 |
| Hemoglobin, mean (SD), mg/dL | 13.9 (2.18) | 14.4 (2.3) | 0.18 |
| Leukocytes×109/L, mean (SD) | 11.4 (10.7) | 10.0 (3.5) | 0.20 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in 1 second; SatO2, blood oxygen; SD, standard deviation; VTE, venous thromboembolism.
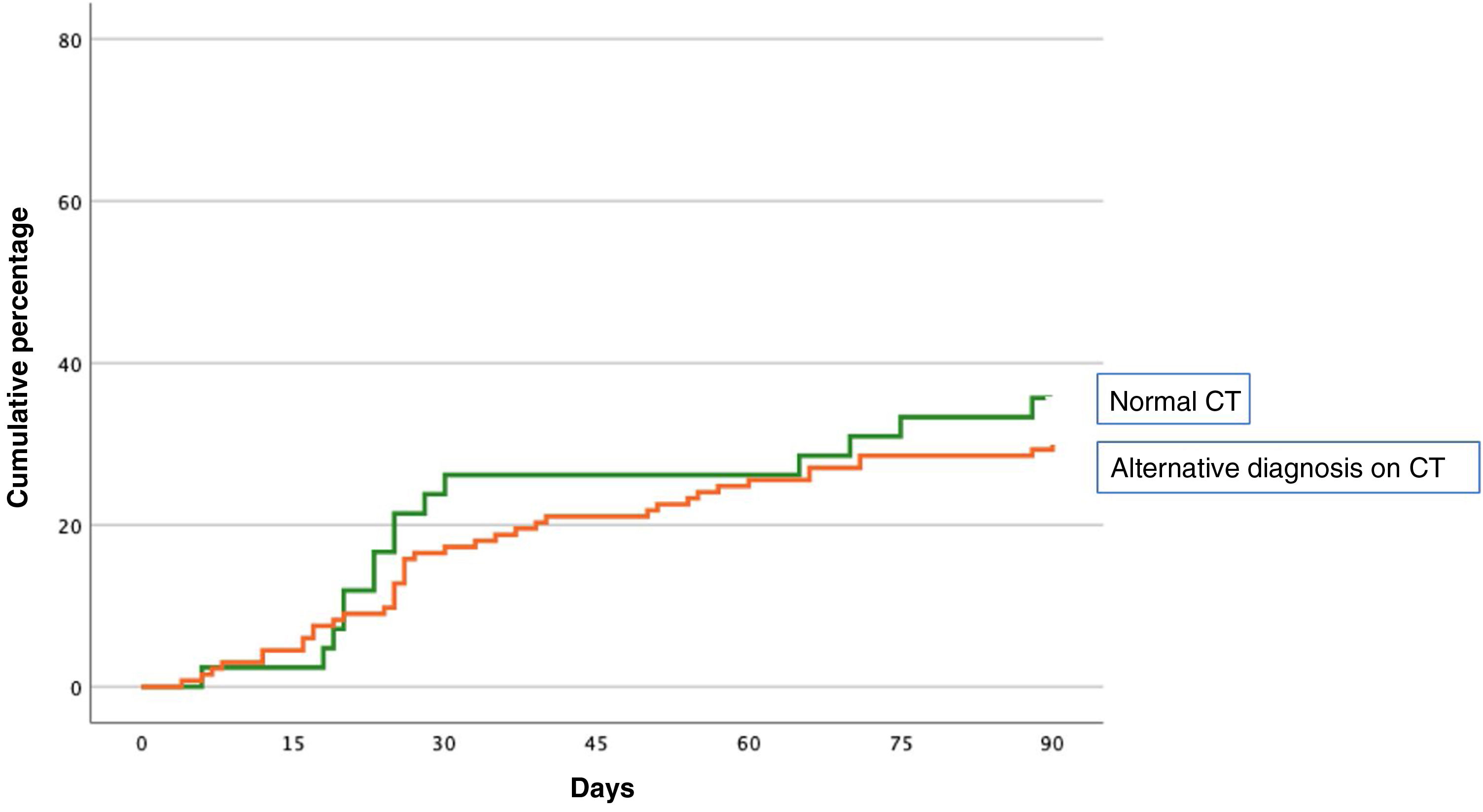
The primary event occurred in 55 (31.4%; 95% CI, 24.6–38.9%) of the 175 patients during the first 90 days of follow-up (Table 2). Forty-five patients (25.7%) were readmitted due to exacerbation of their COPD, and 16 patients (9.1%) died, of whom 6 had previously been readmitted. The primary event occurred in 40 of 133 patients (30.1%) with an alternative diagnosis to PE on chest CT, and in 15 of 42 patients (35.7%) with normal CT (difference 5.6%, 95% CI, −10.6 to 23.6%) (Fig. 1). Table 2 shows the distribution of events according to the presence or absence of an alternative diagnosis to PE on chest CT.
Events during 90-day follow-up.
| Events | All patients(N=175) | With alternative diagnosis on CT(N=133) | With normal CT(N=42) |
|---|---|---|---|
| Primary event | |||
| Readmission for COPD or all-cause death | 55 (31.4) | 40 (30.1) | 15 (35.7) |
| Secondary events | |||
| Readmission for COPD | 45 (25.7) | 30 (22.6) | 15 (35.7) |
| All-cause death | 16 (9.1) | 16 (12.0) | 0 (0) |
| Recurrence of non-fatal thrombosis | 0 (0) | 0 (0) | 0 (0) |
| Hospital length of stay mean (SD), days | 6.9 (4.2) | 6.9 (4.3) | 6.8 (4.2) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; SD, standard deviation.
In the univariate analysis, age>75 years (OR: 1.88; 95% CI: 0.99–3.59; P=0.06), cancer (OR: 4.78; 95% CI: 1.15–19.87; P=0.03), and immobilization for medical reasons (OR: 1.99; 95% CI: 0.96–4.16; P=0.07) significantly increased the risk of the composite endpoint occurring within 90 days of randomization (Table 3). In the multivariate analysis, the finding of an alternative diagnosis to PET on chest CT (versus normal CT) was not independently associated with the composite endpoint in the first 90 days of follow-up (ORadjusted: 0.64; 95% CI: 0.30–1.38; P=0.26) (Table 3).
Univariate and multivariate analysis.
| Predictor variable | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age>75 years | 1.88 (0.99–3.59) | 0.06 | 1.88 (0.96–3.68) | 0.06 |
| Men | 1.56 (0.68–3.57) | 0.30 | – | – |
| Postbronchodilator FEV1 | 0.41 (0.07–2.39) | 0.32 | – | – |
| CHF | 1.94 (0.88–4.25) | 0.10 | – | – |
| Cancer | 4.78 (1.15–19.87) | 0.03 | 5.57 (1.29–24.04) | 0.02 |
| Alternative diagnosis on CT | 0.77 (0.37–1.61) | 0.49 | 0.64 (0.30– 1.38) | 0.26 |
| Immobilization | 1.99 (0.96–4.16) | 0.07 | 2.03 (0.95–4.37) | 0.07 |
| Heart rate>100/min | 1.50 (0.77–2.94) | 0.24 | – | – |
| Systolic blood pressure<100mm Hg | 0.35 (0.04–3.0) | 0.34 | – | – |
| SatO2<90% | 0.89 (0.46–1.72) | 0.72 | – | – |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in 1 second; OR, odds ratio; SatO2, blood oxygen.
After adjusting for age>75 years, cancer, and medical immobilization, an alternative diagnosis to PE on chest CT (versus normal CT) was not independently associated with all-cause mortality (difference 12.0%; 95% CI, 0.6–19.1%; P=.06) or readmission for COPD (ORadjusted: 0.48; 95% CI, 0.22–1.03; P=0.06) within 90 days of randomization.
Secondary analysisWe then analyzed the prognostic significance of the presence (versus absence [i.e., normal CT]) of any diagnosis, including PE, on chest CT. A specific CT finding was identified in 150 patients (150 of 192 patients; 78.1%; 95% CI, 71.6–83.8%). The finding of a specific diagnosis on chest CT was not independently associated with the composite endpoint (ORadjusted: 0.61; 95% CI: 0.29–1.30; P=0.20), all-cause death (difference 11.3%; 95% CI, 0.0–17.8%; P=0.07), or readmission for COPD (ORadjusted: 0.48; 95% CI: 0.22–1.03; P=0.06) within 90 days of randomization.
DiscussionIn this subgroup analysis of the SLICE clinical trial, an alternative diagnosis to PE was found in 75% of patients with COPD exacerbation who underwent chest CT scan. Although the presence of an alternative diagnosis to PE led to a change in treatment in 23% of cases, it was not associated with lower rate of mortality or readmission for COPD during follow-up. Similar results were obtained when we analyzed the effect of any specific chest CT-based diagnosis (including PE) on the prognosis of these patients.
Several authors have evaluated the frequency of alternative diagnoses in patients undergoing chest CT for suspected PE, and reported an incidence that ranges from 25% to 52%, depending on the study.15–18 In a recent prospective study in 203 patients who underwent chest CT for suspected PE,18 an alternative diagnosis was found in 88 patients (43%), but this only affected therapy in 10 patients (4.9%). There is far less information on the utility of chest CT in patients hospitalized for COPD exacerbation. In a retrospective study in 202 patients hospitalized for a COPD exacerbation, a specific diagnosis was made in 42.1% of patients undergoing CT scan.12 Although these findings led to a change in treatment in 10.9% of patients, length of hospital stay and need for intensive care were similar in both groups. Our results confirm these findings. Although a specific diagnosis is often found on chest CT, this leads to a change in treatment in very few cases, and has no significant effect on prognosis. Clinical practice guidelines suggest the use of oral corticosteroids and antibiotics for COPD exacerbations that require hospital admission.19 For this reason, more than three quarters of our patients with an alternative diagnosis to PE on chest CT were already receiving specific treatment for that diagnosis, and this could explain why chest CT findings did not modify prognosis in our study.
Our results have practical consequences. It is common for the emergency department to request a chest CT for patients with COPD exacerbation in order to confirm/rule out PE as the cause and to arrive at an alternative diagnosis that could change the patient's existing treatment.20 The SLICE trial showed that an algorithm (including D-dimer and chest CT for D-dimer-positive patients) for PE screening in patients hospitalized for COPD exacerbation had no clinical benefit.11 This subgroup analysis complements those results, and suggests that a chest CT should not be routinely requested in this group of patients.
This study has several limitations. First, it is a predefined subgroup analysis of patients in the intervention group of a clinical trial who underwent a chest CT scan. The results, therefore, are merely intended to generate hypotheses. Although adjustments were not made for multiplicity, the absence of an independent association between an alternative diagnosis on CT and the primary outcome variable rules out a type I error and makes such adjustment unnecessary. Second, the number of events was too low to draw strong conclusions. Finally, in the SLICE trial, only patients in the intervention group with positive D-dimer results underwent chest CT, so our results cannot be extrapolated to all patients with COPD exacerbation requiring hospital admission.
In conclusion, chest CT in patients requiring admission for COPD exacerbation provides an alternative diagnosis (other than PE) in 76% of cases. Specific treatment of these pathologies does not improve prognosis in the first 3 months of follow-up.
Authors’ contributionsConcept and design: Rodriguez, Jimenez.
Data acquisition, data analysis and interpretation: Rodríguez, Solier, Marín, Tenes, Durán, Retegui, Muriel, Otero, Monreal, Jiménez.
Writing of the manuscript: Rodriguez, Solier, Marin, Jimenez.
Supervision: Rodriguez, Jimenez.
The corresponding author, David Jiménez, had full access to the study data, and was ultimately responsible for submitting the manuscript for publication.
FundingISC III (PI14/00400), Chest Foundation, Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), Neumosur and Daiichi Sankyo.
Conflict of interestsNone.
Barcelona (20 patients): S. Jiménez (16 patients), A. Vilas (3 patients), D. Aisa (1 patient); Bilbao (113 patients): E. Tabernero/B. González-Quero (113 patients); Galdakao (21 patients): A. Ballaz/L. Chasco (21 patients); Gran Canaria (35 patients): G. Pérez-Peñate/F. León-Marrero (35 patients); La Coruna (25 patients): P. Marcos-Rodriguez/SJ Dominguez-Pazos (25 patients); Madrid (364 patients): D. Jimenez/A. Quezada (171 patients), A. Hernando/JI de Granda-Orive (91 patients), P. Ruiz-Artacho/F. Beddar-Chaib (56 patients), MJ Rodríguez-Nieto/Itziar Fernández-Ormaechea (28 patients), M. Calle/JL Rodriguez-Hermosa/J. Carriel (12 patients), A. Martínez-Verdasco (2 patients), J. de Miguel-Díez (2 patients), MA Quesada (2 patients); Santander (17 patients): – R. Agüero (17 patients); Seville (112 patients): L. Jara-Palomares/R. Otero/E. Marquez-Martín (112 patients); Valencia (8 patients): R. López-Reyes (8 patients); Vitoria (31 patients): J.L. Lobo/A. Rivas-Guerrero (31 patients).