Transbronchial cryobiopsy (TBCB) provides larger tissue samples and improved sampling depth, but its role in diagnosing acute cellular rejection (ACR) in lung transplant patients is unclear due to limitations in existing studies. To address this, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of TBCB.
MethodsA thorough literature review was conducted to evaluate TBCB in post-lung transplant surveillance, assessing the quality of studies and conducting a meta-analysis comparing diagnostic yields of TBCB and transbronchial forceps biopsy (TBFB), as well as evaluating procedural complications.
ResultsOur meta-analysis, incorporating 11 studies with a total of 915 patients, showed that TBCB had a diagnostic rate of 38.27% (225/588) for ACR post-lung transplantation, notably higher than the 35.65% (251/704) for TBFB. The inverse-variance weighted odds ratio was calculated at 2.32 (95% confidence interval: 1.24–4.32; p=0.008). Funnel plot analysis indicated no major publication bias. Meta-analysis of 6 studies demonstrated that TBCB, compared to TBFB, significantly increased the diagnostic rate for chronic rejection post-transplantation (25.00% vs 10.93%, p=0.005). Our meta-analysis comparing the safety of TBCB and TBFB in post-lung transplant surveillance found no significant differences in moderate to severe bleeding (5.99% vs 6.31%, p=0.98), or pneumothorax incidence (3.90% vs 3.29%, p=0.75).
ConclusionsOur study indicates that TBCB may enhance the diagnosis of acute and chronic rejection post-lung transplantation with a safety profile comparable to TBFB. Further research and the development of standardized procedures are warranted to ensure the safe and effective application of TBCB in broader clinical practice.
In most clinical scenarios, bronchoscopy procedures such as transbronchial lung biopsy (TBLB) and bronchoalveolar lavage (BAL) are typically sufficient to diagnose acute cellular rejection (ACR) in lung transplant recipients, often making surgical lung biopsy unnecessary for diagnostic confirmation.1–3 Yet, the limited sample size and presence of extrusion artifacts in tissue samples from transbronchial forceps biopsy (TBFB) present significant challenges for pathologists in confidently diagnosing ACR.4 This limitation is evidenced in the literature by the low sensitivity of TBFB for this condition.5 Although BAL is effective in identifying infections, its efficacy in conclusively diagnosing rejection is less clear.6 Conversely, while surgical biopsy yields an ample amount of pathological tissue for detailed analysis, its invasive nature increases the risk of secondary infection, delayed wound healing, and other complications associated with immunosuppression, which in turn limits its clinical utility.
In recent years, transbronchial cryobiopsy (TBCB) has emerged as a viable alternative bronchoscopic procedure for histological sampling in the diagnostic evaluation of lung diseases.7 TBCB has been extensively utilized for the collection of samples from a range of diseases, including lung tumors, interstitial lung diseases, and pulmonary infections,8–10 owing to its capacity to procure larger pathological tissue samples and minimize the occurrence of artifacts. Numerous studies have substantiated the efficacy and safety of this technique, thereby potentially addressing the shortcomings associated with TBFB and surgical lung biopsy.11,12 However, due to the limited data currently available on the safety and efficacy of post-lung transplantation monitoring, the application value of TBCB in ACR after lung transplantation, especially in terms of safety, remains controversial compared to TBFB.13–18 The conclusions regarding the incidence rates of moderate to severe bleeding and pneumothorax for these two techniques vary significantly across different studies.
A prior meta-analysis examined the utility of TBCB in monitoring patients after lung transplantation, indicating that compared to TBFB, this procedure yields more tissue samples and fewer artifacts.19 However, the meta-analysis did not address the diagnostic efficacy and safety of TBCB in detecting acute cellular rejection (ACR) in lung transplant recipients. Additionally, the value of the meta-analysis for guiding clinical practice is limited as it is based solely on a conference abstract that includes four studies. After conducting a review of the literature, it was found that a number of additional original studies have been published subsequent to the aforementioned meta-analysis. Due to the variability in reporting methods among these original studies, the shortcomings of the prior meta-analysis, and the influx of new research in this field, it was deemed necessary to conduct a new meta-analysis. Therefore, the aim of this meta-analysis is to incorporate newly published literature for a comprehensive and thorough review, assessing the effectiveness and safety of TBCB compared to TBFB in diagnosing ACR post-lung transplantation.
Materials and methodsThis study conducted a meta-analysis of existing data in accordance with the guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses statement (PRISMA-DTA).20 As a result, ethics committee approval was deemed unnecessary. Furthermore, the meta-analysis has been registered in PROSPERO under the registration number CRD42024513485 (https://www.crd.york.ac.uk/prospero/).
Search strategyA comprehensive literature search was carried out in the PubMed, EMBASE, Web of Science, and Scopus databases from their inception up to February 11, 2024. The search strategy included the terms “cryobiopsy,” “cryoprobe biopsy,” and “lung transplantation” OR “lung allograft,” which were applied to titles and abstracts. Furthermore, references of the included articles were examined for additional pertinent studies, and conference abstracts were consulted to identify unpublished research. The full texts of all selected studies were meticulously reviewed to ascertain their adherence to the PICOS (population, intervention, comparison, outcome, study design) criteria. The research involved a cohort of post-lung transplant individuals undergoing bronchoscopy, with interventions comprising TBCB and TBFB. The control group was comprised of patients who solely underwent TBFB, and the study assessed diagnostic yield and complications as outcome measures.
Inclusion criteria and exclusion criteriaIn the present meta-analysis, randomized controlled trials and observational studies were incorporated to compare the efficacy of TBCB and TBFB in detecting ACR following lung transplantation. These studies evaluated the diagnostic yield of both biopsy methods in diagnosing ACR post-transplantation, while also documenting any associated complications. Case reports or series with fewer than four subjects, lung transplantation studies utilizing only TBCB or TBFB for diagnosing ACR of lung transplantation, as well as studies with non-standardized procedures and duplicate data were all excluded from the analysis.
Data extraction and outcomes assessedIn order to streamline the management of literature, the retrieved results were imported into EndNote20 software for the purpose of eliminating duplicate literature and conducting initial screening. The eligibility of included papers was independently evaluated by two reviewers (SP Li and Y Luo) based on predetermined inclusion and exclusion criteria. Upon determining that the screened literature met the criteria for inclusion, pertinent data such as the first author's name, year of publication, age of participant, design of study, criteria for selection, and other relevant outcomes were extracted from the articles.
The primary aim of this meta-analysis was to assess the diagnostic efficacy of TBCB compared to TBFB in cases of ACR following lung transplantation. The secondary objectives included evaluating the diagnostic efficacy of TBCB versus TBFB in cases of chronic rejection after lung transplantation, as well as analyzing the safety profile of TBCB relative to TBFB.
Quality assessmentThe quality assessment of the studies was conducted by two authors (SP Li and Y Luo) independently, with any discrepancies resolved through consensus discussions. The methodological quality of the observational studies included in the analysis was assessed utilizing the Newcastle–Ottawa Scale,21 which considers three key components: selection of patient, comparability of study groups, and assessment of exposure. Each study was evaluated and given a numerical score on a scale of 0–9, with a score of 6 or higher denoting high quality and a score below 6 denoting low quality. The quality of the randomized controlled trials included in the analysis was evaluated using the Jadad scale,22 which comprises three components: randomization (0–2 points), blinding (0–2 points), and withdrawals (0–1 points). Studies with a score equal to or greater than 3 was categorized as high quality, while a score below 3 was considered low quality.
DefinitionsThe diagnostic criteria23 for acute and chronic cellular rejection were applied according to the revised guidelines for pulmonary rejection published in 1996. Bleeding grading24 was categorized as severe if bronchial blocking or embolization was necessary, moderate if bleeding ceased with epinephrine or cold saline treatment, and mild if it could be stopped spontaneously or with continuous airway suction. To aid in assessing clinically significant bleeding events, moderate and severe cases were pooled for analysis.
Statistical analysisThe meta-analysis and statistical analysis in this study utilized Cochrane RevMan 5.4 software and Stata 15 software. The diagnostic positive rate of each study was aggregated using the inverse variance weighting method, and the odds ratio (OR) was subsequently calculated. Heterogeneity among the studies was assessed using the Cochran Q test and I2 statistic. A fixed-effects model was employed when statistical heterogeneity was low (I2<50%, p>0.10), while a random-effects model was utilized otherwise. Publication bias was evaluated using Egger's test to assess funnel plot asymmetry, with a significance level set at 0.05.
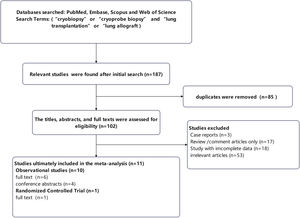
ResultsSearch resultsThe methodology employed for study selection is depicted in Fig. 1. A comprehensive search of PubMed, EMBASE, Web of Science, and Scopus yielded 187 distinct titles and abstracts, from which duplicates were eliminated, resulting in 102 studies for preliminary review. Subsequently, the title, abstract, or full text of each of the 102 studies underwent detailed scrutiny in accordance with predetermined inclusion and exclusion criteria. Contact with the corresponding author was considered as a means to procure any necessary data. Fifty-three studies were excluded from this meta-analysis due to lack of alignment with the research focus. Three studies were excluded as they were case reports, while eighteen studies were excluded due to insufficient data. Furthermore, seventeen studies were excluded for being solely review or comment articles.
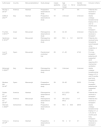
Study characteristics and qualitiesIn the systematic review we conducted, 11 studies13–18,25–29 were incorporated, including 7 full-text articles and 4 conference abstracts. This ensemble consists of 10 cohort studies and 1 randomized controlled trial. Within the cohort study, the distribution is balanced, with 5 prospective and the remaining 5 retrospective. A cumulative total of 915 patients were encompassed within the 11 studies analyzed (sample size range: 4 to 402). This cohort included 834 patients reported in seven full-text publications and 81 patients from four conference abstracts, as detailed in Table 1. In this systematic review, the included studies primarily feature work from authors based in Europe and North America. Every study encompassed in this systematic review compared the effectiveness of monitoring with TBCB versus TBFB in post-lung transplant patients. Age data was captured in nine of these studies, with the reported mean or range spanning from 20 to 65.5 years. Eight studies provided gender distribution, indicating a male predominance with percentages varying between 44% and 75%.
Characteristic features of included studies in the present meta-analysis.
| Author/year | Country | Manuscript/abstract | Study design | Cases (patients) | Age (mean±SD or range) | Gender (male/female) | Inclusion criteria |
|---|---|---|---|---|---|---|---|
| Akulian J 201227 | America | Abstract | Prospective observational study | 10 | 57 | 5/5 | Patients who received a transplant |
| Daffrè E 202125 | Italy | Abstract | Prospective observational study | 54 | Unknown | Unknown | Adults undergo transbronchial biopsy at 3, 6, and 12 months post lung transplant |
| Fruchter O 201319 | Israel | Manuscript | Retrospective observational study | 40 | 42–64 | Unknown | Patients who received a transplant |
| Gershman E 201824 | Israel | Manuscript | Retrospective observational study | 402 | 53.6±13.1 | 242/160 | Patients who received lung transplants were biopsied using cryoprobe or forceps |
| Loor K 202329 | Spain | Manuscript | Randomized controlled trial | 89 | 41–62 | 47/42 | For lung transplant patients with suspected ACR requiring ICU mechanical ventilation |
| Mohamed S 202028 | Italy | Manuscript | Retrospective observational study | 164 | Unknown | Unknown | Adults undergo transbronchial biopsy at 3, 6, and12 months post lung transplant |
| Montero MA 201822 | Spain | Manuscript | Prospective observational study | 58 | 20–65 | 35/23 | For lung transplant patients with suspected ACR |
| Roden AC 201520 | America | Abstract | Retrospective observational study | 13 | 61.0 (25.2–65.5) | 8/5 | Patients who received a transplant |
| Roden AC 201623 | America | Manuscript | Retrospective observational study | 18 | 48.4 (25.2–64.8) | 11/7 | Patients who received a transplant |
| Steinack C 202221 | Switzerland | Manuscript | Prospective observational study | 63 | 56.4±8.83 | 28/35 | Adults undergo transbronchial biopsy at 1, 2, 4, 6 and 12 months post lung transplant |
| Yarmus L 201226 | America | Abstract | Prospective observational study | 4 | 53±12 | 3/1 | Patients who received a transplant |
Abbreviations: TBCB, transbronchial lung cryobiopsy; TBFB, transbronchial forceps biopsy.
The methodologies and tools used in the studies are detailed in Table 2. Of the studies included, seven recorded the size of the cryoprobes used, with dimensions being 1.7mm, 1.9mm, and 2.4mm. Additionally, seven studies noted the freezing time of the cryoprobes, which ranged from 3 to 7s. Eight studies provided data on the number of TBCB and TBFB conducted, with TBCB ranging from 2 to 6 times and TBFB ranging from 2 to 10 times. Out of the 11 studies analyzed, 9 studies indicated that TBCB yielded a greater specimen volume compared to TBFB. Different studies used different metrics to measure size. Additionally, 4 of these studies found that TBCB specimens exhibited fewer artifacts than TBFB specimens.
The methods and materials of included studies in the present meta-analysis.
| Author/year | Cryoprobe size | Freezing time | Number of TBCB | Number of TBFB | Specimen size of TBCB | Specimen size of TBFB | Artifacts of TBCB | Artifacts of TBFB |
|---|---|---|---|---|---|---|---|---|
| Akulian J 201227 | Unknown | Unknown | 5 | 10 | 57.9±11.3mm2 | 12.9±4.8mm2 | Unknown | Unknown |
| Daffrè E 202125 | Unknown | Unknown | 6 | 3 | Unknown | Unknown | Unknown | Unknown |
| Fruchter O 201319 | 2.4mm | 4s | 2–3 | 6–8 | 10 (5–20.1)mm2 | 2 (0.5–4)mm2 | Unknown | Unknown |
| Gershman E 201824 | 2.4mm | 4s | 2–3 | 4–6 | 16.6mm2 | 6.6mm2 | 0 | 11 |
| Loor K 202329 | 1.9/2.4mm | 3s | Unknown | Unknown | 3.45±1.2mm | 2.23±1.12mm | 4 | 13 |
| Mohamed S 202028 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Montero MA 201822 | 2.4mm | 3s | Unknown | Unknown | 22.1±12.5mm2 | 8.5±6.5mm2 | 0 | 9 |
| Roden AC 201520 | 1.9/2.4mm | Unknown | 1.3 (on average) | 1.3 (on average) | 0.456 (0.256–3.071)cm3 | 0.096 (0.035–0.472)cm3 | Unknown | Unknown |
| Roden AC 201623 | 1.9/2.4mm | 3–5s | 3 | 2 | 0.50(0.06–3.07)cm3 | 0.13(0.02–0.64)cm3 | 8 | 26 |
| Steinack C 202221 | 1.7/2.4mm | 4–7s | 2 | 5 | 10.1±7.1mm | 2.3±1.8mm | Unknown | Unknown |
| Yarmus L 201226 | Unknown | 3–5s | 5 | 10 | 31.3(16–60)mm2 | 9.7(0.15–25)mm2 | Unknown | Unknown |
Abbreviations: TBCB, transbronchial lung cryobiopsy; TBFB, transbronchial forceps biopsy.
The quality assessment of the seven full-text studies included in our meta-analysis is summarized in Supplementary Table 1, showing varied levels of quality. Of the six observational studies, five were rated as high quality and one as low quality. The included prospective randomized controlled trial was also evaluated and found to be of high quality. Due to limited information, the quality of the four conference abstracts could not be determined.
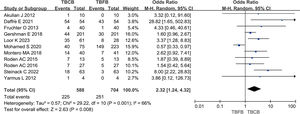
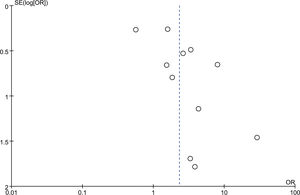
Diagnostic value of TBCB and TBFBThe meta-analysis, encompassing 11 studies, revealed that the diagnostic rate of TBCB for ACR following lung transplantation was 38.27% (225/588). Conversely, the diagnostic rate for TBFB stood at 35.65% (251/704). The analysis yielded an inverse-variance weighted odds ratio of 2.32 (95% confidence interval: 1.24–4.32; p=0.008), with a heterogeneity index of 66%. Fig. 2 depicts these findings in a forest plot. Additionally, the funnel plot presented in Fig. 3 indicated an absence of significant publication bias (Egger's test, p=0.09).
Furthermore, the meta-analysis of six studies indicated that the diagnostic rate of TBCB for chronic rejection after lung transplantation was 25.00% (68/272), compared to 10.93% (46/421) for TBFB. The variance inverse-weighted odds ratio was determined to be 3.18 (95% confidence interval: 1.65–6.13), with a heterogeneity index (I2) of 0% (p=0.005). These results are visually represented in Supplementary Fig. 1 through forest plots. Additionally, the funnel plot in Supplementary Fig. 2 did not indicate any significant publication bias (Egger's test, p=0.546).
ComplicationsSignificant bleeding and pneumothorax are common complications in patients monitored with TBCB following lung transplantation. The meta-analysis of ten studies revealed the incidence of moderate to severe hemorrhage to be 5.99% (32/534) in the TBCB group and 6.31% (41/650) in the TBFB group, showing no statistically significant difference (p=0.98) (Supplementary Fig. 3). Additionally, the analysis of eight studies demonstrated that the incidence of pneumothorax was 3.90% (18/461) in the TBCB group versus 3.29% (19/577) in the TBFB group, again with no significant difference (p=0.75) (Supplementary Fig. 4).
DiscussionLung transplantation is a critical therapeutic intervention for patients with end-stage lung diseases, aimed at extending survival and improving quality of life.30 In contemporary medical practice, this procedure is primarily indicated for conditions like interstitial lung disease and chronic obstructive pulmonary disease. A notable hurdle faced by postoperative recipients is ACR, with approximately 27% of recipients experiencing rejection within the initial year following transplantation.31 The association of ACR with chronic rejection and its potential to adversely affect prognosis highlights the importance of its timely and accurate detection, as any delays in diagnosis could compromise graft function.32 Therefore, the implementation of effective detection methods for ACR is essential in refining treatment strategies and enhancing patient outcomes.
The diagnosis of lung transplant rejection is primarily dependent on microbiological and pathological data obtained through bronchoscopy.33 Given the limitations of TBFB and BAL, TBCB is increasingly acknowledged as a valuable alternative for post-lung transplantation monitoring. It is acclaimed for its innovative, safe, and effective approach, enabling the collection of larger tissue samples without compromising morphological integrity, thereby reducing the need for repeated procedures.34 In recent years, TBCB has gained increasing popularity among interventional pulmonologists, marking significant progress in this field. However, despite these advancements, there appears to be some differing opinions regarding the efficacy and especially the safety of TBCB compared to TBFB for post-lung transplantation monitoring. The incidence rates of moderate to severe bleeding and pneumothorax associated with TBCB and TBFB vary considerably across different studies. Therefore, a systematic review and meta-analysis to provide a comprehensive evaluation of the diagnostic efficacy and safety of TBCB for ACR after lung transplantation could potentially be of significant clinical value. A previous meta-analysis included only four studies and did not specifically examine the role of TBCB in diagnosing ACR, which suggests the necessity for a more thorough and updated meta-analysis.
In our systematic review and meta-analysis, we carefully evaluated the potential effectiveness and safety of TBCB in comparison to TBFB for monitoring patients after lung transplantation. The findings suggest that TBCB may be more effective in diagnosing ACR than TBFB. Additionally, for chronic rejection diagnosis post-transplantation, TBCB appears to have a higher likelihood of effectiveness compared to TBFB. The generally superior quality of TBCB samples, characterized by their larger size and deeper extraction, along with fewer artifacts, might facilitate earlier and more accurate detection of rejection, potentially leading to better patient outcomes following a transplant. In terms of safety, our gathered data on complications following TBCB and TBFB procedures showed no significant differences in terms of moderate to severe bleeding and pneumothorax events between the groups. Based on these findings, TBCB might be considered a potentially safe and effective alternative to TBFB for postoperative monitoring in lung transplant patients.
The present study has gathered a thorough selection of relevant literature for quality evaluation and meta-analysis, with the intention of delivering a more comprehensive and objective assessment of the diagnostic role of TBCB in ACR. To the best of our understanding, our meta-analysis concerning TBCB for post-lung transplant surveillance possibly represents the most substantial sample size to date. Given the growing interdisciplinary interest in cryo-technology within pulmonology and thoracic surgery, and the current lack of multicenter randomized trial data on this subject, our study is both timely and crucial for advancing our understanding of the role of TBCB in post-transplant lung tissue sampling.
It should be noted that although the data incorporated into the meta-analysis suggest promising progress in the effectiveness and safety of TBCB for detecting rejection after lung transplantation, further issues may need to be clarified before it is considered a routine monitoring method for lung transplant patients. In clinical practice, thorough preoperative examinations (including echocardiography, coagulation function tests, blood routine tests, etc.) are advisable to identify risk factors such as bleeding tendency and pulmonary hypertension before performing TBCB under bronchoscopy.29,35 Additionally, enhancing formal training for bronchoscopists could help reduce the incidence of adverse events, given that the diagnostic accuracy and safety of TBCB appear to be closely related to professional expertise and the standardization of technical procedures.36 It is also worth considering whether the current research results from a few large centers are applicable to other centers, especially those with fewer resources and less experience.
We should also recognize the limitations of this meta-analysis. Firstly, the number of existing studies on this topic is somewhat limited, and the heterogeneity among them might affect the statistical power and generalizability of our findings, suggesting a cautious interpretation of the results. Additionally, as most of the included studies are observational, there could be selection bias and confounding factors influencing the outcomes. Furthermore, since all the studies were conducted at single centers, the lack of standardization and multicenter data may pose challenges to the reliability of our meta-analysis results. Therefore, we advocate for the initiation of multicenter studies and the establishment of standardized protocols to more robustly validate the efficacy of TBCB in post-lung transplantation applications.
ConclusionIn conclusion, our analysis of the limited available studies suggests that TBCB enhances the diagnostic rate of ACR and chronic rejection following lung transplantation when compared to TBFB. Moreover, there appears to be no significant difference in the incidence of complications between TBCB and TBFB. However, further research and the development of standardized procedures are warranted to ensure the safe and effective application of TBCB in broader clinical practice.
Statement of ethicsIn this meta-analysis, the research process didn’t involve new human subject data since it is based on an aggregation and analysis of existing published studies. As a result, ethics committee approval was deemed unnecessary.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsYan Luo was tasked with the acquisition, analysis, and interpretation of data, as well as drafting the initial manuscript. Sheng-ping Li handled the development of the conceptual framework and the bibliographic review, and critically revised vital intellectual content. All co-authors were actively involved in revising and giving their final approval to the manuscript.
Conflict of interestsThe authors declare no competing interests.
Data availabilityAll relevant data can be found within the articles included in this study and in the supplementary materials.
Artificial intelligence involvementThis study was conducted without the aid of any artificial intelligence software or tools.
NA.