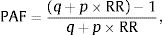In 2021, children (aged <15 years) accounted for 12% (1.2 million) of all tuberculosis (TB) new cases globally, half of which were in patients under 5 years of age.1 First-line treatment of pediatric TB is based on a combination of 3–4 anti-TB oral drugs (isoniazid [H], rifampicin [R], pyrazinamide [Z], and ethambutol [E]) in the intensive phase (2 months); and H and R for 2–4 extra months in the consolidation phase in most cases.2 In 2010, the WHO decided to increase the recommended doses of first-line anti-TB oral drugs for children, based on pharmacokinetic studies that demonstrated insufficient serum peak concentrations (Suppl. Table 1).3–6 Anti-TB treatment in children is well tolerated, gastrointestinal disturbances and hepatotoxicity being the most commonly reported adverse events (AE), all of them associated with H, R and Z use.7 Data on the toxicity of the increased recommended doses are still scarce. We aimed to determine the toxicity rates and severity of the new recommended doses of first-line anti-TB oral drugs in young children in Spain.
We conducted an observational retrospective study in a cohort of children treated for TB at a referral pediatric center in Spain from 2014 to 2022. All children diagnosed with TB and due to start first-line oral treatment, with weights ≤30kg at diagnosis, in whom H dose would be doubled as compared to previous recommendations, were eligible. In our center, children weighing >30kg are treated as per adult dose protocols and were not affected by the WHO recommendation on increased dosages; therefore, they were excluded from the study. Those patients referred after having initiated anti-TB treatment elsewhere or with incomplete follow-up, those receiving 2nd line anti-TB drugs, and those with an underlying condition and/or receiving other chronic treatments that could represent a confusion bias were also excluded. The study was approved by the local Ethics Committee (ref. PIC 103-16). Informed consent was obtained from parents or legal guardians at inclusion.
The diagnosis of TB was based on epidemiological, clinical, radiological, and microbiological findings, according to published consensus criteria,8 and was classified according to location (intrathoracic and/or extrathoracic), severity,9 and as microbiologically-confirmed or not. All patients received anti-TB treatment on a daily basis as per national protocols.2 Adherence to anti-TB treatment was defined as taking at least 85% of daily doses and was reinforced through a nurse-led educational intervention and assessed by means of a written questionnaire.10 Clinical AE were documented during anti-TB treatment, and laboratory data were obtained at baseline and at the end of the intensive phase (2 months into anti-TB treatment). AE severity was graded according to DAIDS classification.11 Causality between AE and anti-TB drug use was categorized as unlikely, possible, probable or definite. The rates of treatment failure, TB recurrence, TB-associated sequelae and death from any cause, as well as the time elapsed from the end of anti-TB treatment and last available follow-up visit were calculated. All statistical analyses were performed using SPSS V29 (IBM; Armond, NY), with statistical significance defined as a p-value <0.05.
Overall, 155 patients were diagnosed with TB during the study period, none of them HIV co-infected. Of these, 92 children fulfilled the inclusion criteria and 63 were excluded (Fig. 1 and Suppl. Table 2). Median (interquartile range, IQR) anti-TB treatment duration was 27 (26–28) weeks, with adequate adherence in all cases; all patients were successfully treated. Twenty-nine and 9 clinical AE were noted in 14 and 7 patients in the intensive and the consolidation phase, respectively, mostly consisting of gastrointestinal distress (63%; Table 1). They were all mild, except for a brief resolved unexplained event in a 2-year-old girl that required admission and that was unlikely related to anti-TB treatment; this consisted in a sudden episode of loss of consciousness and hypotonia with normal vital signs and no trigger, that recovered within minutes; extensive diagnostic workup was negative and the episode has not recurred thereafter. Five patients (5.4%) experienced 2 and 7 adverse events that were probably and possibly associated with anti-TB therapy; the former consisted in vomiting one hour after medication intake, and a thrush. All patients complaining of gastrointestinal symptoms had normal alanine aminotransferase (ALT) levels. No patient complained of symptoms consistent with E-related optic neuritis.
Flow-chart showing the patients that were screened, the reasons for exclusion, and the main characteristics of the tuberculosis disease in the 92 patients that were included. 1Family migration to other cities/countries in 8 cases, and one adolescent that ran away from shelter and was lost to follow-up. 2Acute lymphoblastic leukemia and severe combined immunodeficiency that required allogeneic bone-marrow transplantation, n=1 each.
Spectrum of the Clinical Adverse Events That Were Observed During the Intensive and the Consolidation Phases of Anti-tuberculosis Treatment. Note That Some Patients Experienced >1 Adverse Event.
| Adverse Event | n | Severity11 | Causality | Outcome |
|---|---|---|---|---|
| Intensive phase | ||||
| Vomiting | 3 | Mild | Unlikely | Resolved with antiemetic drugs |
| 1 | Unlikely | Self-limited | ||
| 1 | Possible | Stopped treatment for 3 days | ||
| 1 | Probable | Self-limited | ||
| Abdominal pain | 5 | Mild | Unlikely | Self-limited |
| 1 | Unlikely | Resolved with omeprazol | ||
| 1 | Possible | Stopped treatment for 3 days | ||
| Nausea | 1 | Mild | Possible | Self-limited |
| 1 | Possible | Stopped treatment for 3 days | ||
| Diarrhea | 1 | Mild | Possible | Resolved with probiotics |
| Perianal exanthema | 2 | Mild | Possible | Self-limited |
| Stomatitis | 2 | Mild | Unlikely | Self-limited |
| Thrush | 1 | Mild | Probable | Resolved with miconazole |
| Balanitis | 1 | Mild | Unlikely | Resolved with proper hygiene habits |
| Upper respiratory tract infection | 2 | Mild | Unlikely | Resolved with symptomatic treatment |
| Fever | 2 | Mild | Unlikely | Resolved with paracetamol |
| 1 | Possible | Stopped treatment for 3 days | ||
| Febrile seizure | 1 | Mild | Unlikely | Self-limited |
| Brief resolved unexplained event | 1 | Severe | Unlikely | Self-limited |
| Consolidation phase | ||||
| Vomiting | 1 | Mild | Unlikely | Self-limited |
| Abdominal pain | 1 | Mild | Unlikely | Self-limited |
| Watery diarrhea | 1 | Mild | Unlikely | Self-limited |
| Gingivitis | 1 | Mild | Unlikely | Resolved with proper hygiene habits |
| Feet desquamation | 1 | Mild | Unlikely | Self-limited |
| Urinary tract infection | 1 | Mild | Unlikely | Cured with fosfomycin |
| Upper respiratory tract infection | 1 | Mild | Unlikely | Self-limited |
| Febrile seizure | 1 | Mild | Unlikely | Self-limited |
| Headache | 1 | Mild | Unlikely | Resolved with paracetamol |
Despite the over time significant changes in many laboratory parameters, most of them remained within normal limits both at baseline and at the end of the intensive phase (Suppl. Table 3). Eight (8.7%) patients showed mildly elevated non-symptomatic ALT levels, probably caused by anti-TB therapy, but that did not require dose adjustment or interruption of therapy. ALT levels normalized in all the former patients at the end of anti-TB treatment. Mild non-symptomatic elevation of urates (<10mg/dL) was observed in 26 (28.3%) children and was definitely caused by Z. Severe neutropenia (<100cells/mm3) during the consolidation phase was identified in a 3-month-old girl; this was possibly related to anti-TB treatment, which had to be interrupted for one month. The patient did not develop secondary infections and could complete anti-TB therapy without incidence. Neither the occurrence of clinical AE nor elevated ALT levels during anti-TB treatment were associated with baseline demographic or clinical variables or the dose per kg of anti-TB drugs (data not shown). After 3.8 (median values; IQR=2.0–6.5) years of follow-up after the end of anti-TB treatment, no TB recurrences or deaths were observed. Four patients (4.3%) affected with TB meningitis developed long-term sequelae.
In 2010, the increased dose recommendations of first-line of anti-TB oral drugs for the treatment of pediatric TB were rapidly implemented worldwide. Since then, several studies have reported on their toxicity in high-TB-endemic low-income countries.12–14 Spain is a low-TB-endemic country (7.6 cases per 100,000 inhabitants in 2021),15 where TB in children is often diagnosed earlier in contact tracing studies, and patients are usually less symptomatic at TB diagnosis and less affected with other comorbidities, such as malnutrition or HIV infection, all these factors putting them at lower risk of treatment failure or toxicity. In this scenario, the outcomes were excellent, as previously reported.16 Mild self-limited gastrointestinal distress (vomiting, diarrhea, abdominal pain, anorexia, etc.) unrelated to hepatotoxicity remains the most frequent AE associated with first-line anti-TB drugs, also in our cohort.7,12–14 These are very unspecific and common signs and symptoms, especially in young children, and it is often very difficult to definitely establish causality. Z-associated rashes or optic neuritis upon E exposure have been reported, but are very rare in the pediatric age.7
With regards to laboratory parameters, the decrease in leukocyte, neutrophil and platelet counts, and in inflammatory markers, as well as the increase in hemoglobin and albumin levels we observed can be attributed to an improvement in the inflammatory response as a result of anti-TB treatment. Most importantly, mild non-symptomatic elevated ALT levels (less than 2 times the upper limit of normal, ULN) were not uncommon (8.7%) during the intensive phase, but no single case of H-associated hepatitis (3 times the ULN if with symptoms, or 5 times the ULN without symptoms) was observed. This is reassuring and consistent with previous reports, both with the previous doses17 and with the newly-recommended increased doses.12–14 It is known that H-associated hepatitis is idiosyncratic, not clearly related to dose or duration of therapy, and occurs in <1% of children. Also, R and Z can cause hepatitis, albeit less frequently.7 Therefore, ALT monitoring during anti-TB therapy is not recommended in previously healthy children in the lack of symptoms.7 Finally, the non-symptomatic increase in urates levels is very frequent with Z exposure, as the drug promotes its reabsorption in the renal tubules.18 This rarely associates symptoms in children, and some authors have even suggested that it can be used as a surrogate marker of proper adherence to treatment.19
Our observational study has several limitations, including the small sample size; its retrospective nature, which carries a risk of missing data; the lack of directed observed treatment that did not allow optimal assessment of adherence; and the difficulty of establishing causality between drug exposure and the AE. Also, we did not perform systematic opportunistic screening of children receiving E, which may have led to an underdiagnosis of mild optic neuritis cases. Visual evaluation is very difficult in young children.20
In summary, the increased dosage of first-line oral anti-TB drugs recommended by the WHO in 2010 is well tolerated in children and is not associated with a higher risk of hepatotoxicity or other AE.
FundingThis work was partially supported by a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), reference PI22/00766, and by the Spanish Society of Pneumology and Thoracic Surgery, grant number 169-2022.
Conflicts of InterestThe authors state that they have no conflict of interests.
This study constituted the Final Degree Project of author Laura PEREIRA at Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, Barcelona, Spain.