Clinical practice guidelines recommend risk stratification of patients with pulmonary embolism (PE), as it has important implications for the therapeutic management.1–3 They also suggest close monitoring of patients with intermediate-high risk PE, and prompt reperfusion (beyond standard anticoagulation) if decompensation occurs soon after diagnosis and initiation of anticoagulant therapy.1 Echocardiographic evaluation of the right ventricle (RV) function/size plays a crucial role in the prognostication of patients with acute PE.4 However, among patients with acute intermediate-risk PE, the time course for recovery of echocardiographic RV dysfunction and thus the role of echocardiography to determine the duration of intensive monitoring lack clarity.5 Therefore, we aimed to assess the temporal dynamics of echocardiographic RV dysfunction, and whether normalization of echocardiographic findings was associated with clinical outcomes.
We pooled the patients from the control groups of two randomized trials, the AntiINflamatorios para el tratamiento de la Embolia de Pulmón submasiva (AINEP) trial and the Air versus oxygen for Intermediate-Risk pulmonary embolism (AIR) trial.6,7 In brief, both trials enrolled patients with intermediate-risk PE (i.e., normal blood pressure and echocardiographic RV dysfunction), who were randomized to receive diclofenac plus anticoagulation vs anticoagulation alone, or supplemental oxygen plus anticoagulation vs ambient air plus anticoagulation, respectively. Trained and certified local cardiologists, blinded to the patient's clinical data and the time of the echocardiography, interpreted each echocardiogram, and these measurements were used for analyses. RV dysfunction was defined as a RV diameter greater than the left ventricle diameter in the apical or subcostal view; and/or RV free wall hypokinesis (assessed by qualitative interpretation of systolic RV free wall motion). The primary analysis examined the proportion of patients who had resolution of echocardiographic RV dysfunction at 48h after randomization. The secondary analysis was the resolution of RV dysfunction at 7 days after randomization. Comparisons of continuous data were performed with the two-sided paired t test. Ordinal data were compared with the McNemar's test.
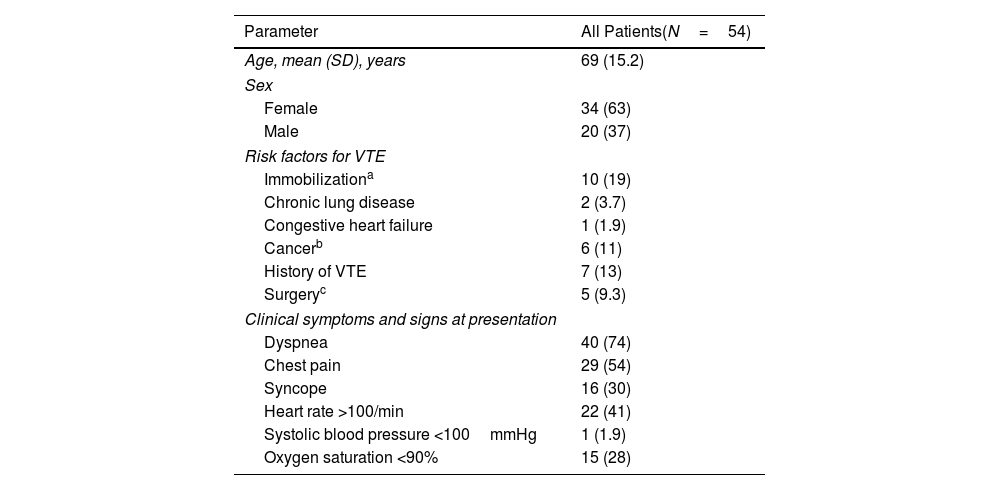
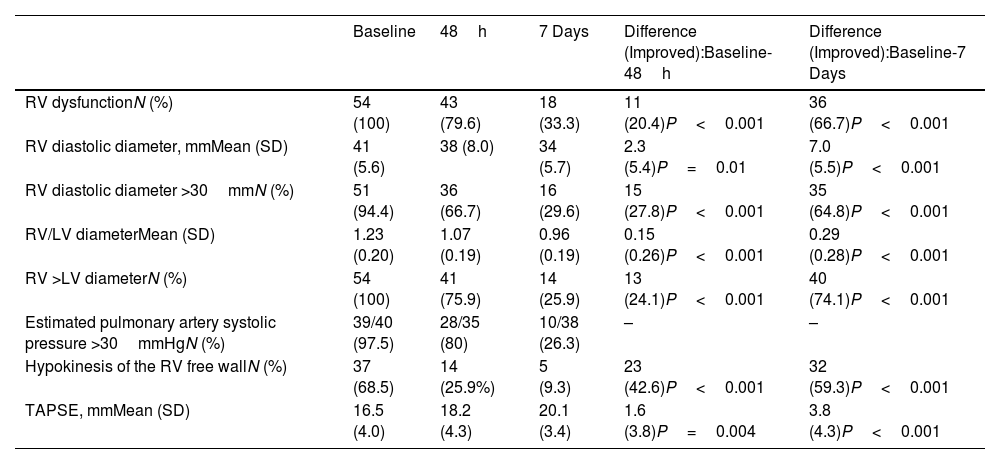
The study cohort consisted of 54 haemodynamically stable patients (mean [SD] age, 69 [15.2] years; 34 women [63%]) with RV dysfunction at the time of PE diagnosis who received standard anticoagulation (Table 1). At baseline, the mean RV end-diastolic diameter and the RV/LV ratio were 41 [5.6] mm and 1.23 [0.20], respectively. Mean tricuspid annular plane systolic excursion (TAPSE) was 16.5 [4.0] mm. At 48h post-randomization, the proportion of patients without RV dysfunction was 20.4% (11/54; 95% confidence interval [CI], 10.6%–33.5%). Of the 43 patients with persistent RV dysfunction at 48h, 41 had RV dilatation and 14 had RV hypokinesis (12 had both persistent RV dilatation and RV hypokinesis). The proportion of patients without RV dysfunction at 7 days was 66.7% (36/54; 95% confidence interval [CI], 52.5%–78.9%). Of the 18 patients with persistent RV dysfunction at 7 days, 14 had RV dilatation and 5 had RV hypokinesis (1 had both persistent RV dilatation and RV hypokinesis). Full dynamics of echocardiographic parameters are shown in Table 2. During follow-up, one (1.9%; 95% CI, 0.1%–9.9%) patient experienced hemodynamic collapse 3 days after randomization, and another one died of cancer. This patient had persistent RV dilatation at day 7.
Baseline Characteristics of the Patients.
| Parameter | All Patients(N=54) |
|---|---|
| Age, mean (SD), years | 69 (15.2) |
| Sex | |
| Female | 34 (63) |
| Male | 20 (37) |
| Risk factors for VTE | |
| Immobilizationa | 10 (19) |
| Chronic lung disease | 2 (3.7) |
| Congestive heart failure | 1 (1.9) |
| Cancerb | 6 (11) |
| History of VTE | 7 (13) |
| Surgeryc | 5 (9.3) |
| Clinical symptoms and signs at presentation | |
| Dyspnea | 40 (74) |
| Chest pain | 29 (54) |
| Syncope | 16 (30) |
| Heart rate >100/min | 22 (41) |
| Systolic blood pressure <100mmHg | 1 (1.9) |
| Oxygen saturation <90% | 15 (28) |
Abbreviations: SD, standard deviation; VTE, venous thromboembolism.
Echocardiographic Data.
| Baseline | 48h | 7 Days | Difference (Improved):Baseline-48h | Difference (Improved):Baseline-7 Days | |
|---|---|---|---|---|---|
| RV dysfunctionN (%) | 54 (100) | 43 (79.6) | 18 (33.3) | 11 (20.4)P<0.001 | 36 (66.7)P<0.001 |
| RV diastolic diameter, mmMean (SD) | 41 (5.6) | 38 (8.0) | 34 (5.7) | 2.3 (5.4)P=0.01 | 7.0 (5.5)P<0.001 |
| RV diastolic diameter >30mmN (%) | 51 (94.4) | 36 (66.7) | 16 (29.6) | 15 (27.8)P<0.001 | 35 (64.8)P<0.001 |
| RV/LV diameterMean (SD) | 1.23 (0.20) | 1.07 (0.19) | 0.96 (0.19) | 0.15 (0.26)P<0.001 | 0.29 (0.28)P<0.001 |
| RV >LV diameterN (%) | 54 (100) | 41 (75.9) | 14 (25.9) | 13 (24.1)P<0.001 | 40 (74.1)P<0.001 |
| Estimated pulmonary artery systolic pressure >30mmHgN (%) | 39/40 (97.5) | 28/35 (80) | 10/38 (26.3) | – | – |
| Hypokinesis of the RV free wallN (%) | 37 (68.5) | 14 (25.9%) | 5 (9.3) | 23 (42.6)P<0.001 | 32 (59.3)P<0.001 |
| TAPSE, mmMean (SD) | 16.5 (4.0) | 18.2 (4.3) | 20.1 (3.4) | 1.6 (3.8)P=0.004 | 3.8 (4.3)P<0.001 |
Abbreviations: RV, right ventricle; SD, standard deviation; LV, left ventricle; TAPSE, tricuspid annular plane systolic excursion.
The results of our study showed that, in patients with intermediate-risk PE, the proportion of patients with echocardiographic RV dysfunction decreased by about 20% within 48h of starting anticoagulant therapy, and by about two thirds within 7 days. These findings are similar to those from a study in which normalization of echocardiographic parameters at 48h occurred in 33% of 85 patients with intermediate-high risk PE who were treated with standard anticoagulation.8 In the Catheter-Directed Thrombolysis vs Anticoagulation in Patients with Acute Intermediate-High-Risk Pulmonary Embolism (CANARY) trial, resolution of RV dilatation at 72h occurred in 24 of the 46 patients (52.2%; 95% CI, 37.0%–67.1%) in the anticoagulation monotherapy arm.9 Moreover, our results are in line with a previous report showing that the resolution of pulmonary embolic obstruction takes approximately one week after initiation of treatment with heparin.10 Therefore, serial echocardiograms that are performed to monitor response to anticoagulation during the acute phase of PE should be interpreted with caution, as a high frequency of false-positive results (i.e., RV dysfunction without significant clinical consequences) is expected under these circumstances.
This is an exploratory analysis with limitations. First, the number of patients with intermediate-risk PE included in this analysis was small, and did not allow for more precision in our estimates. Future analyses that combine individual participant data from different studies might provide more reliable results. Second, all patients received initial therapy with low-molecular-weight heparin, and we could not assess the effect of direct oral anticoagulants on the temporal dynamics of echocardiographic RV dysfunction. Finally, in both trials echocardiograms were done and interpreted by certified local cardiologists and it is not apparent whether using a core laboratory would have yielded similar results.
In conclusion, our findings suggest that a small proportion of patients with intermediate-risk PE normalize RV function/size in the first 2 days after anticoagulant therapy. Our data in the current format should be interpreted as hypothesis generating. Further research is required to elucidate the clinical usefulness of routine serial measurement of echocardiographic parameters to monitor response to anticoagulation in the acute phase of intermediate-risk PE.
Conflict of InterestsDr. Bikdeli is supported by a Career Development Award from the American Heart Association and VIVA Physicians (#938814). Dr. Bikdeli was supported by the Scott Schoen and Nancy Adams IGNITE Award and is supported by the Mary Ann Tynan Research Scientist award from the Mary Horrigan Connors Center for Women's Health and Gender Biology at Brigham and Women's Hospital, and the Heart and Vascular Center Junior Faculty Award from Brigham and Women's Hospital. Dr. Bikdeli reports that he was a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. Dr. Bikdeli has not been involved in the litigation in 2022–2024 nor has he received any compensation in 2022–2024. Dr. Bikdeli reports that he is a member of the Medical Advisory Board for the North American Thrombosis Forum, and serves in the Data Safety and Monitory Board of the NAIL-IT trial funded by the National Heart, Lung, and Blood Institute, and Translational Sciences.