Interstitial lung diseases (ILDs) are a group of fibro-inflammatory lung diseases that occur due to a variety of causes including environmental to genetic factors.1,2 When fibrosis is present, prognosis is typically worsened. The prototypical fibrotic ILD (fILD) is idiopathic pulmonary fibrosis (IPF), nevertheless, fibrosis can be found in a variety of different ILDs.3–5 With the exception of antifibrotics,6 the current management approach to fILD is based on low- or very low-quality evidence, resulting in substantial variation in the standard of care. Confirming or refuting the efficacy of the actual treatments, while also facilitating the development of new therapeutic agents, is an urgent un-met need in the field of fILD.7
Conventional randomized controlled trial (RCT) designs explore a single active therapy over a fixed time making the process slow and inefficient.8 In addition, the field of fILD deals with relatively rare diseases, which increases the challenges of the traditional research entrepreneurship in terms of recruitment and study power.9 Not unexpectedly, there have been few therapeutic successes in fILD and those most penalized by this slow process are our patients.7 Finally, several fILD therapeutic approaches are empirical and variable depending on the country, including the use of glucocorticosteroids and immunosupressors.
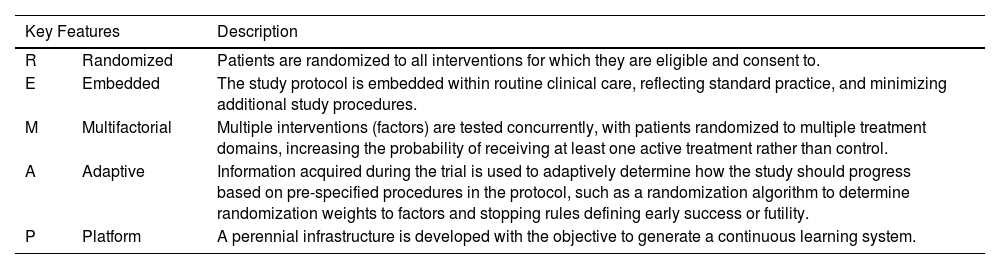
REMAP is an acronym that stands for randomized embedded multifactorial adaptive platform (REMAP), an adaptive platform trial.10 A REMAP design enables numerous existing and novel therapies to be explored for the treatment of fILD, while leveraging a multifactorial analysis model that maximizes the opportunity to quickly identify effective treatments based on high-quality data. Hence, the goal of REMAP-ILD is to accelerate and improve care for patients with fILD using an innovative and efficient REMAP design. REMAPs are called living learning systems,11 and that can be explained by the following characteristics (see Table 1):
- •
Multifactorial design: multiple interventions are tested simultaneously. One patient may be randomized to different interventions helping answer different research questions at once (for example antifibrotics, senolytics, steroids, immunomodulatory drugs etc.).
- •
Embedded: the study is pragmatic and designed to reduce the burden of recruiting centers. REMAP-ILD aims at being embedded in routine clinical care as much as possible.
- •
Adaptive features: REMAP-ILD will use responsive adaptive randomization (RAR) which means that the randomization proportion will change informed by pre-planned interim analyses, favoring the intervention with greatest probability of being the winner. This also increases safety for participating patients without compromising power.
- •
Data analysis: there is no fixed sample size, rather pre-specified thresholds for success and futility are set. These thresholds allow the study to stop when it reaches a threshold rather than to pursue a fixed sample size calculated when no information was available.
- •
Perennial: as a perennial platform, new interventions can enter the platform. As a living learning system, when the conclusion on a particular intervention is of success, that intervention becomes the new standard of care in the platform.
Key Features of REMAP Trials that Increase Efficiency of Research.
| Key Features | Description | |
|---|---|---|
| R | Randomized | Patients are randomized to all interventions for which they are eligible and consent to. |
| E | Embedded | The study protocol is embedded within routine clinical care, reflecting standard practice, and minimizing additional study procedures. |
| M | Multifactorial | Multiple interventions (factors) are tested concurrently, with patients randomized to multiple treatment domains, increasing the probability of receiving at least one active treatment rather than control. |
| A | Adaptive | Information acquired during the trial is used to adaptively determine how the study should progress based on pre-specified procedures in the protocol, such as a randomization algorithm to determine randomization weights to factors and stopping rules defining early success or futility. |
| P | Platform | A perennial infrastructure is developed with the objective to generate a continuous learning system. |
The REMAP-ILD initiative emerged from a shared discontent among ILD researchers and clinicians on the substantial uncertainties that cloud fILD management decisions. A social media platform, X-Twitter, provided the grounds to frequent debates where we all realized the unacceptable lack of data-driven decisions in fILD, the painfully protracted drug development timelines – often up to a decade – and the pressing need for better outcomes for patients with fILD and clinicians. Over time, these discussions matured into a shared realization: if the scientific community, clinicians, and patient partner organizations did not spearhead this ambitious project to speed up solutions for patients living with fILD, no one else would. The critical nature of these life-threatening diseases underscored the urgency of addressing numerous unanswered questions as fast as possible, and this is one reason why REMAP-ILD starts off focusing on *fibrotic* ILDs, the subset of ILDs with worse prognosis. It also became clear that a global collaborative effort was fundamental, considering the relatively rare occurrence of these diseases and therefore the improbability of a single isolated effort triumphing over such complex challenges.
REMAP-ILD was inspired by other successful REMAP, the REMAP-CAP.12 REMAP-CAP is an adaptive platform trial originally designed to address community acquired pneumonia. REMAP-CAP was able to rapidly pivot to COVID-19 delivering answers in a shorter timeframe. The two main features that allowed REMAP-CAP to deliver so efficiently were: 1. the trial platform was in place (perennial) when COVID-19 struck the world and 2. its multifactorial nature, allowing to test various research questions simultaneously.12 REMAP-CAP randomized more than 18,000 patients and addressed various research questions across 18 domains of interventions (steroids, macrolids, vitamin C, immunomodulation, anticoagulation strategies, etc.).12
REMAP-ILD moved from theory to reality: in 2022, founders applied for an accelerator grant in the UK which was successful, enabling the initiation of the trial design work [Kawano-Dourado Thorax, 2024].13
REMAPs allow multiple interventions to be investigated simultaneously using a common control group, hence increasing research efficiency.12,13 Various methodological efficiencies like hierarchical borrowing, response adaptive randomization, repeatable reusable analytical framework further increase efficiency.13 On the other hand, REMAPs are more complex and their design requires extensive expertise. Additionally, REMAPs face challenges in harmonizing regulatory, data protection, ethical, and governance procedures across different regions. Overcoming these challenges requires establishing regional working groups, and engaging them in iterative discussions with regulatory bodies to ensure protocols meet their requirements effectively. Funding constraints pose an additional hurdle for REMAP-ILD (a global network), often restricting study components to specific regions based on available funding. This underscores the necessity for enhanced international collaboration among funding organizations, especially in researching rare diseases like fILD.
The respiratory community of Ibero-American countries has worked together over the years in several programs and activities, especially through the Spanish and Latin-American scientific societies (SEPAR and ALAT). Joint ILD research efforts include the telomere study in IPF patients,14 SEPAR-ALAT state of the art in progressive pulmonary fibrosis,15 the Annual Ibero-American colloquium for research networking SEPAR-ALAT, and the more recent Ibero-American registry of hypersensitivity pneumonitis (REGINHA). Therefore, the network structure for joining REMAP-ILD is already set and the Ibero-American ILD community could easily embrace this worldwide initiative.
The benefits to Ibero-American patients are short and long-term. In the short term, REMAP-ILD offers the opportunity to patients to access medications not yet available in some countries (for example antifibrotics). It also broadens the opportunity for patients with fILD, a life-threatening disease, to have access to clinical trials. In the long-term, REMAP-ILD will enhance the collaborative Ibero-American research structure which directly affects patient care through the positive impact of clinical research in clinical practice. The Ibero-American community will also be able to contribute with the mission of the REMAP-ILD: increasing representativity and diversity in the patient population recruited while helping REMAP-ILD deliver the answers patients need.
Through worldwide collaboration, novel analytic methodology, and pragmatic trial delivery, REMAP-ILD aims to overcome major limitations associated with conventional RCT approaches to improve the care of people living with fILD. The integration of Ibero-American ILD community could be a great opportunity for global research and clinical care for our patients.
This initiative also presents an opportunity to enhance access to innovative treatments, advance scientific research, and improve the management of interstitial lung diseases in Ibero-American countries.
Conflicts of InterestLKD reports research grants from Boehringer Ingelheim, Bristol-Myers-Squibb, a research grant from the Brazilian Ministry of Health (PROADI-SUS), non-financial research support from Fisher & Paykel, personal fees from Boehringer Ingelheim. JS reports research grants from Boehringer Ingelheim and Roche, personal fees from Boehringer Ingelheim, Atyr, Aflorfarm and Roche. MMM reports research grants and fees for scientific advise from Boehringer Ingelheim, Roche, Ferrer, Chiesi. JS reports research grants from Boehringer Ingelheim and Roche, personal fees from Boehringer Ingelheim, Atyr, Aflorfarm and Roche. JIE reports research and educational grants from Boehringer Ingelheim, and personal fees from Boehringer Ingelheim, Roche, Bristol-Myers-Squibb, Bagó and Raffo pharmaceutical Companies.