Bronchodilator response (BDR) assessed by forced expiratory volume in 1 second (FEV1) is used in routine clinical practice to diagnose respiratory diseases such as chronic obstructive pulmonary disease (COPD) and/or asthma, assess asthma control, and to predict the response to inhaled treatment.1 Clinical characteristics such as higher fractional exhaled nitric oxide (FeNO), wheezing, and allergic sensitization have been directly associated with BDR, while age and body mass index (BMI) present an inverse association.2 Degree of BDR is positively related to severe, uncontrolled disease and higher asthma-related mortality.3 Death was found to be 7 times more likely among patients with over 50% BRD.3 Therefore, bronchodilator response may have a significant impact on clinical course, management, and symptoms and has been suggested as a potentially modifiable risk factor for exacerbations.1,4 Nevertheless, BRD is widely influenced by anti-inflammatory asthma treatment.5
This study aimed to describe the frequency of positive and negative BDR using two different criteria in a large real-life cohort of asthmatic patients and to analyze patient clinical characteristics. As secondary objectives, we evaluated the associations of BDR with asthma control and severity, frequency of exacerbations, and inflammatory phenotypes.
This prospective observational study was conducted by reviewing the MEGA cohort electronic database, a well-characterized real-life cohort of asthmatic patients of several severities.6 Data on asthma diagnosis and study variables have been described previously.6 Positive BDR was defined as recommended by ERS/ATS 20227 [a change of >10% in (post-bronchodilator value (L)−pre-bronchodilator value (L))×100/predicted value (L)] and also using the former criteria, ERS/ATS 19918 (≥12% and ≥200ml increase in FEV1 after administration of 200μg salbutamol). The Ethics Committees of each participating hospital approved this study. All subjects provided signed informed consent.
Lung function parameters: FEV1, forced vital capacity, FVC, FEV1/FVC, total lung capacity, TCL and residual volume, RV were converted to z-scores for each subject using the Global Lung Initiative reference values (GLI)9 and were divided into 3 groups according to their pattern10: air trapping (z score for FVC<−1.64 or a change in FVC with bronchodilation of ≥10% predicted) obstructive (z-score for FEV1/FVC<−1.64), and normal (FVC z>−1.64 and FEV1/FVC z>−1.64).
Quantitative variables were described as mean and standard deviation, and qualitative variables by absolute and relative frequencies. Inter-group comparisons were performed using the Chi-square test or Fisher's exact test for qualitative and ANOVA or Kruskal–Wallis test for quantitative variables. Area under receiving operator curves (AUC) and odds ratios were also obtained in variables with a significant difference. Agreement between both classifications was measured using Kappa index. The corresponding 95% confidence interval (95% CI) was also computed. Statistical analysis was carried out using GraphPad Instat6 (GraphPad Software Inc., San Diego, CA). p-Values<0.05 were considered significant.
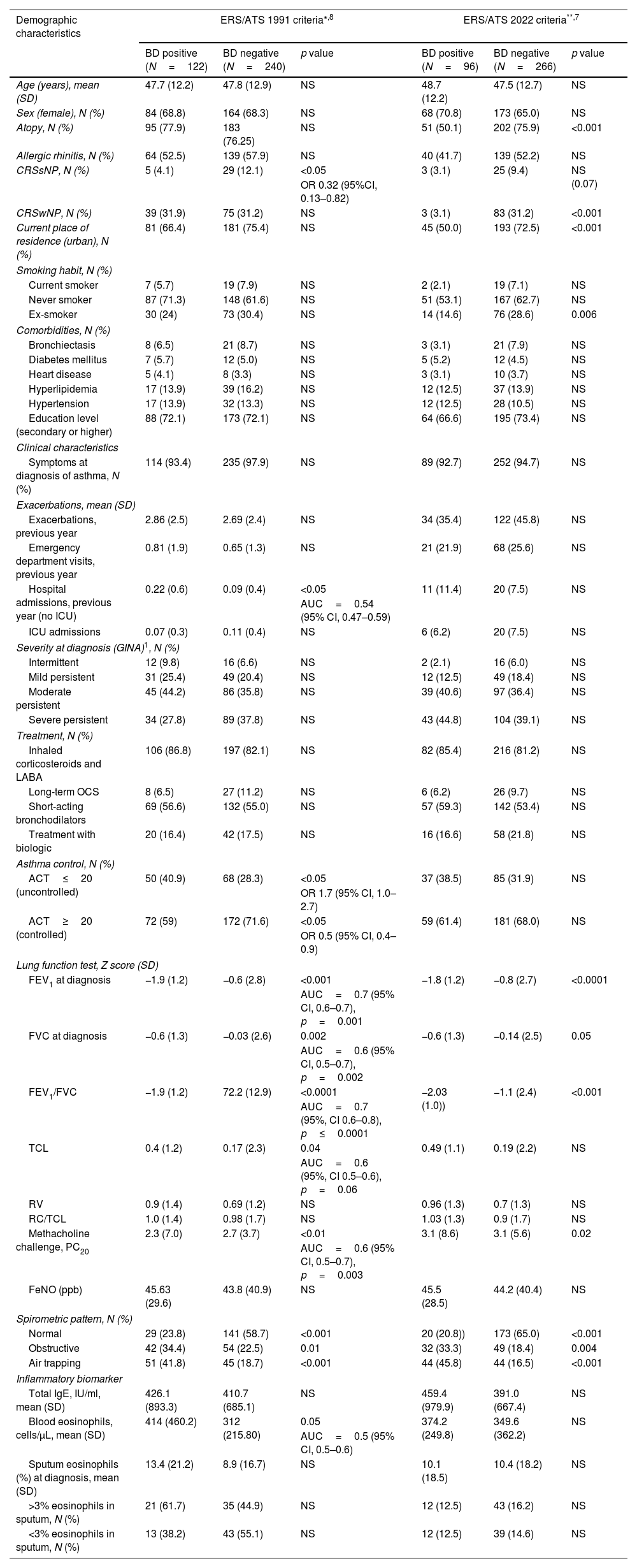
Off the 512 subjects from the MEGA cohort,6 362 had complete data about spirometric parameters and reversibility and were selected in our study. The cohort was divided according to the results of spirometry BDR from the initial visit. Mean age was 47.8±12.5 years, and 67% were female. Most of the patients were receiving asthma treatment. Ninety-six (26.5%) and 122 (33.7%) patients had positive BDR according with ERS/ATS-2022 and 1991, respectively. Overall, 88 patients coincided with positive bronchodilation according to both criteria and in 226 with negative results. Kappa agreement was κ=0.75 [IC95%=(0.68, 0.83)]. There were no significant differences between patients with positive and negative BDR using both criteria in most of the analyzed items (see Table 1). A significant association of positive BDR using both criteria was only found with lower FEV1, FVC and FEV1/FVC and methacholine PC20, but not with RV, TLC or RV/TLC ratio, an index of lung hyperinflation measured by whole-body plethysmography. Of note, chronic rhinosinusitis without nasal polyps (CRSsNP) was more frequent in patients with negative BDR (p=0.02) using the former criteria and chronic rhinosinusitis with nasal polyps (CRSwNP) using the new one only (p<0.001). Compared demographic data are summarized in Table 1.
Demographic and clinical characteristics of the studied asthma patient's cohort.
| Demographic characteristics | ERS/ATS 1991 criteria*,8 | ERS/ATS 2022 criteria**,7 | ||||
|---|---|---|---|---|---|---|
| BD positive (N=122) | BD negative (N=240) | p value | BD positive (N=96) | BD negative (N=266) | p value | |
| Age (years), mean (SD) | 47.7 (12.2) | 47.8 (12.9) | NS | 48.7 (12.2) | 47.5 (12.7) | NS |
| Sex (female), N (%) | 84 (68.8) | 164 (68.3) | NS | 68 (70.8) | 173 (65.0) | NS |
| Atopy, N (%) | 95 (77.9) | 183 (76.25) | NS | 51 (50.1) | 202 (75.9) | <0.001 |
| Allergic rhinitis, N (%) | 64 (52.5) | 139 (57.9) | NS | 40 (41.7) | 139 (52.2) | NS |
| CRSsNP, N (%) | 5 (4.1) | 29 (12.1) | <0.05 | 3 (3.1) | 25 (9.4) | NS (0.07) |
| OR 0.32 (95%CI, 0.13–0.82) | ||||||
| CRSwNP, N (%) | 39 (31.9) | 75 (31.2) | NS | 3 (3.1) | 83 (31.2) | <0.001 |
| Current place of residence (urban), N (%) | 81 (66.4) | 181 (75.4) | NS | 45 (50.0) | 193 (72.5) | <0.001 |
| Smoking habit, N (%) | ||||||
| Current smoker | 7 (5.7) | 19 (7.9) | NS | 2 (2.1) | 19 (7.1) | NS |
| Never smoker | 87 (71.3) | 148 (61.6) | NS | 51 (53.1) | 167 (62.7) | NS |
| Ex-smoker | 30 (24) | 73 (30.4) | NS | 14 (14.6) | 76 (28.6) | 0.006 |
| Comorbidities, N (%) | ||||||
| Bronchiectasis | 8 (6.5) | 21 (8.7) | NS | 3 (3.1) | 21 (7.9) | NS |
| Diabetes mellitus | 7 (5.7) | 12 (5.0) | NS | 5 (5.2) | 12 (4.5) | NS |
| Heart disease | 5 (4.1) | 8 (3.3) | NS | 3 (3.1) | 10 (3.7) | NS |
| Hyperlipidemia | 17 (13.9) | 39 (16.2) | NS | 12 (12.5) | 37 (13.9) | NS |
| Hypertension | 17 (13.9) | 32 (13.3) | NS | 12 (12.5) | 28 (10.5) | NS |
| Education level (secondary or higher) | 88 (72.1) | 173 (72.1) | NS | 64 (66.6) | 195 (73.4) | NS |
| Clinical characteristics | ||||||
| Symptoms at diagnosis of asthma, N (%) | 114 (93.4) | 235 (97.9) | NS | 89 (92.7) | 252 (94.7) | NS |
| Exacerbations, mean (SD) | ||||||
| Exacerbations, previous year | 2.86 (2.5) | 2.69 (2.4) | NS | 34 (35.4) | 122 (45.8) | NS |
| Emergency department visits, previous year | 0.81 (1.9) | 0.65 (1.3) | NS | 21 (21.9) | 68 (25.6) | NS |
| Hospital admissions, previous year (no ICU) | 0.22 (0.6) | 0.09 (0.4) | <0.05 | 11 (11.4) | 20 (7.5) | NS |
| AUC=0.54 (95% CI, 0.47–0.59) | ||||||
| ICU admissions | 0.07 (0.3) | 0.11 (0.4) | NS | 6 (6.2) | 20 (7.5) | NS |
| Severity at diagnosis (GINA)1, N (%) | ||||||
| Intermittent | 12 (9.8) | 16 (6.6) | NS | 2 (2.1) | 16 (6.0) | NS |
| Mild persistent | 31 (25.4) | 49 (20.4) | NS | 12 (12.5) | 49 (18.4) | NS |
| Moderate persistent | 45 (44.2) | 86 (35.8) | NS | 39 (40.6) | 97 (36.4) | NS |
| Severe persistent | 34 (27.8) | 89 (37.8) | NS | 43 (44.8) | 104 (39.1) | NS |
| Treatment, N (%) | ||||||
| Inhaled corticosteroids and LABA | 106 (86.8) | 197 (82.1) | NS | 82 (85.4) | 216 (81.2) | NS |
| Long-term OCS | 8 (6.5) | 27 (11.2) | NS | 6 (6.2) | 26 (9.7) | NS |
| Short-acting bronchodilators | 69 (56.6) | 132 (55.0) | NS | 57 (59.3) | 142 (53.4) | NS |
| Treatment with biologic | 20 (16.4) | 42 (17.5) | NS | 16 (16.6) | 58 (21.8) | NS |
| Asthma control, N (%) | ||||||
| ACT≤20 (uncontrolled) | 50 (40.9) | 68 (28.3) | <0.05 | 37 (38.5) | 85 (31.9) | NS |
| OR 1.7 (95% CI, 1.0–2.7) | ||||||
| ACT≥20 (controlled) | 72 (59) | 172 (71.6) | <0.05 | 59 (61.4) | 181 (68.0) | NS |
| OR 0.5 (95% CI, 0.4–0.9) | ||||||
| Lung function test, Z score (SD) | ||||||
| FEV1 at diagnosis | −1.9 (1.2) | −0.6 (2.8) | <0.001 | −1.8 (1.2) | −0.8 (2.7) | <0.0001 |
| AUC=0.7 (95% CI, 0.6–0.7), p=0.001 | ||||||
| FVC at diagnosis | −0.6 (1.3) | −0.03 (2.6) | 0.002 | −0.6 (1.3) | −0.14 (2.5) | 0.05 |
| AUC=0.6 (95% CI, 0.5–0.7), p=0.002 | ||||||
| FEV1/FVC | −1.9 (1.2) | 72.2 (12.9) | <0.0001 | −2.03 (1.0)) | −1.1 (2.4) | <0.001 |
| AUC=0.7 (95%, CI 0.6–0.8), p≤0.0001 | ||||||
| TCL | 0.4 (1.2) | 0.17 (2.3) | 0.04 | 0.49 (1.1) | 0.19 (2.2) | NS |
| AUC=0.6 (95%, CI 0.5–0.6), p=0.06 | ||||||
| RV | 0.9 (1.4) | 0.69 (1.2) | NS | 0.96 (1.3) | 0.7 (1.3) | NS |
| RC/TCL | 1.0 (1.4) | 0.98 (1.7) | NS | 1.03 (1.3) | 0.9 (1.7) | NS |
| Methacholine challenge, PC20 | 2.3 (7.0) | 2.7 (3.7) | <0.01 | 3.1 (8.6) | 3.1 (5.6) | 0.02 |
| AUC=0.6 (95% CI, 0.5–0.7), p=0.003 | ||||||
| FeNO (ppb) | 45.63 (29.6) | 43.8 (40.9) | NS | 45.5 (28.5) | 44.2 (40.4) | NS |
| Spirometric pattern, N (%) | ||||||
| Normal | 29 (23.8) | 141 (58.7) | <0.001 | 20 (20.8)) | 173 (65.0) | <0.001 |
| Obstructive | 42 (34.4) | 54 (22.5) | 0.01 | 32 (33.3) | 49 (18.4) | 0.004 |
| Air trapping | 51 (41.8) | 45 (18.7) | <0.001 | 44 (45.8) | 44 (16.5) | <0.001 |
| Inflammatory biomarker | ||||||
| Total IgE, IU/ml, mean (SD) | 426.1 (893.3) | 410.7 (685.1) | NS | 459.4 (979.9) | 391.0 (667.4) | NS |
| Blood eosinophils, cells/μL, mean (SD) | 414 (460.2) | 312 (215.80) | 0.05 | 374.2 (249.8) | 349.6 (362.2) | NS |
| AUC=0.5 (95% CI, 0.5–0.6) | ||||||
| Sputum eosinophils (%) at diagnosis, mean (SD) | 13.4 (21.2) | 8.9 (16.7) | NS | 10.1 (18.5) | 10.4 (18.2) | NS |
| >3% eosinophils in sputum, N (%) | 21 (61.7) | 35 (44.9) | NS | 12 (12.5) | 43 (16.2) | NS |
| <3% eosinophils in sputum, N (%) | 13 (38.2) | 43 (55.1) | NS | 12 (12.5) | 39 (14.6) | NS |
ERS/ATS 1991 criteria: positive if ≥12% and ≥200ml increase in FEV1 after administration of 200μg salbutamol.
ERS/ATS 2022 criteria: positive if a change of >10% in post-bronchodilator value (L)−pre-bronchodilator value (L)×100/predicted value (L).
ACT, asthma control test; AUC, area under the curve; BD, bronchodilator; CRSwNP, chronic rhinosinusitis with nasal polyposis; CRSsNP, chronic rhinosinusitis without nasal polyposis; FEV1, forced expiratory volume in one second; FeNO, fractional exhaled nitric oxide; FVC, forced vital capacity; IC, interval of confidence; ICU, intensive care unit; IgE, immunoglobulin E; LABA, long-acting β2-receptor agonists; N, sample size; NS, non-significant; OCS, oral corticosteroids; OR, odds ratio; ROC, receiver operating characteristic; PC20, provocative concentration of methacholine causing a 20% fall in FEV1; RV, residual volume; SD, standard deviation; TLC, total lung capacity.
Uncontrolled asthma measured by asthma control test (ACT) (40.9% vs 28.3%, p=0.025), higher hospital admission (0.2 vs 0.09, p=0.028) and higher blood eosinophilia [(414.4 vs 312.0cell/μL, p=0.023, AUC (0.5, p=0.02)], were associated with positive BDR by the former criteria but not for the new one. Obstructive and air trapping pattern were associated with positive BDR in both classifications (p<0.05) No other inflammatory biomarkers demonstrated statistically significant associations (see Table 1 for details).
In this real-life cohort, only 26% of patients with asthma exhibited positive BDR using the new recommendation of ERS/ATS 2022, and 33% using ERS/ATS 1991 BDR criteria. Nevertheless, the agreement between both criteria was high. Thus, that means that new and former criteria coexist in many patients, as expected due to the characteristics of the formula employed. We can only compare the prevalence of BDR with previous studies using 1991 criteria. In this case, our results are similar to other published studies.11,12 However, this rate is higher than the 17% rate reported by the other 3 European cohorts of asthmatic patients, probably due to differences in characteristics among the populations studied.2 Despite both BDR criteria are of limited value for the diagnosis of asthma, especially in patients with normal spirometry and in asthma treatment. The new criteria consider each patient's individual characteristics.7 Measuring the change relative to the predicted value instead of a standard cut point increases specificity to detect lung function changes and allows personalized and individual-guided treatment. In our study, positive BDR with both criteria were significantly associated only with low spirometric values (FVC, FEV1, FEV1/FVC) and lower methacholine PC20. However, RV/TLC ratio, an index of lung hyperinflation, was not associated with BDR status in our study. Hyperinflation is not common in asthma and is associated with severe disease,15 and this feature is of limited use in diagnosing asthma even when baseline spirometry appears normal.
When comparing with other studies done with the ERS/ATS 1991 criteria our results depicted that uncontrolled asthma was related to positive BDR as previously described,2,4,11,15 though not with asthma severity, treatment or exacerbation rate, which have been negatively associated in some studies.2,4,11 Regarding inflammatory biomarkers, our study demonstrated significantly higher blood eosinophil counts in patients with positive BRD, which is consistent with other studies,4,13 but not with the findings of Miller et al.9 Other biomarkers of T2-high inflammation such as sputum eosinophilia and FeNO have been positively related to positive BDR,2,4,11 which contrasts with our results.
Concerning the multimorbidity with CRS, CRSsNP, a more frequent non-T2 disease,14 was associated with negative BDR (p=0.02) using the former ERS/ATS 1991 criteria but with CRSwNP and atopy (both p<0.001) using the new one. This fact is difficult to explain, considering the lack of association with other T2 biomarkers.
One limitation was the reduced patients selected in the study from the total of MEGA cohort, 30.3% of the patients were excluded for not having post-bronchodilatation lung function. By protocol, bronchodilatation was not mandatory because methacholine was mandatory (for asthma diagnosis); that is the main reason. We are aware that this may induce some bias. However, our results are like previous studies using the old formula, so the bias should be minimal. Many asthmatic patients share the 2022 and the 1991 criteria of BDR in asthma patients, as demonstrated in this large real-life asthma cohort. Associations of clinical characteristics or inflammatory biomarkers with negative or positive BDR are not interchangeable between the new and old criteria. Only low spirometric and methacholine values are associated with positive BDR using both criteria. Further studies using the new recommendation are needed to confirm our results.
Conflict of interestDra Betancor D is supported by a Rio Hortega Research Contract from Instituto Carlos III, Ministry of Science. Dra Valverde have received fee for lecture from GSK and is part of the advisory board for Organon. Dr. Rial reports personal fees from GSK, Allergy Therapeutics, AstraZeneca outside the submitted work. Dr. González Barcala reports personal fees from ALK, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Laboratorios Esteve, Menarini, Mundipharma, Novartis, Rovi, Roxall, Stallergenes-Greer, Teva, and Grants from Mundipharma outside the submitted work. Dr. Quirce reports personal fees from AstraZeneca, Novartis, Sanofi, Boehringer Ingelheim, Teva, ALK, Mundipharma, GSK, Chiesi, Leti, outside the submitted work. Dr. Soto-Retes reports non-financial support from CIBER de Enfermedades Respiratorias (CIBERES), during the conduct of the study; personal fees from Stallergennes-Greer, Menarini, Novartis, personal fees from GSK, Hal Allergy, Allergy Therapeutics, AstraZeneca, grants from Sociedad Española de Alergología e Inmunología Clínica SEAIC, and Sociedad Española de Neumología y Cirugía Torácica SEPAR, outside the submitted work. Dr. Martinez Rivera reports grants and personal fees from AstraZeneca, Teva, GSK, Novartis, Mundipharma, outsider the submitted work. Dr. Munoz reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Teva, Mundifarma, Chiesi, Faes, outside the submitted work. Dr. Sastre reports grants and personal fees from Sanofi, GSK, Novartis, AstraZeneca, Mundipharma, Faes Farma, outside the submitted work. Dr. Olaguibel reports grants from Sanofi and/or personal fees from AstraZeneca, Mundipharma, outside the submitted work. Dr. Plaza reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Merck, Chiesi, Novartis, Menarini, Sanofi, outside the submitted work. Dr. Mullol reports personal fees and others from Sanofi-Genzyme & Regeneron, Novartis, Viatris (Mylan pharma), Uriach group, Mitsubishi-Tanabe, Menarini, UCB, Astrazeneca, GSK, MSD outside the submitted work. Dra. Del Pozo reports personal fees and others from Sanofi, Astrazeneca, Gsk outside the submitted work. Other authors have no conflicts of interests.