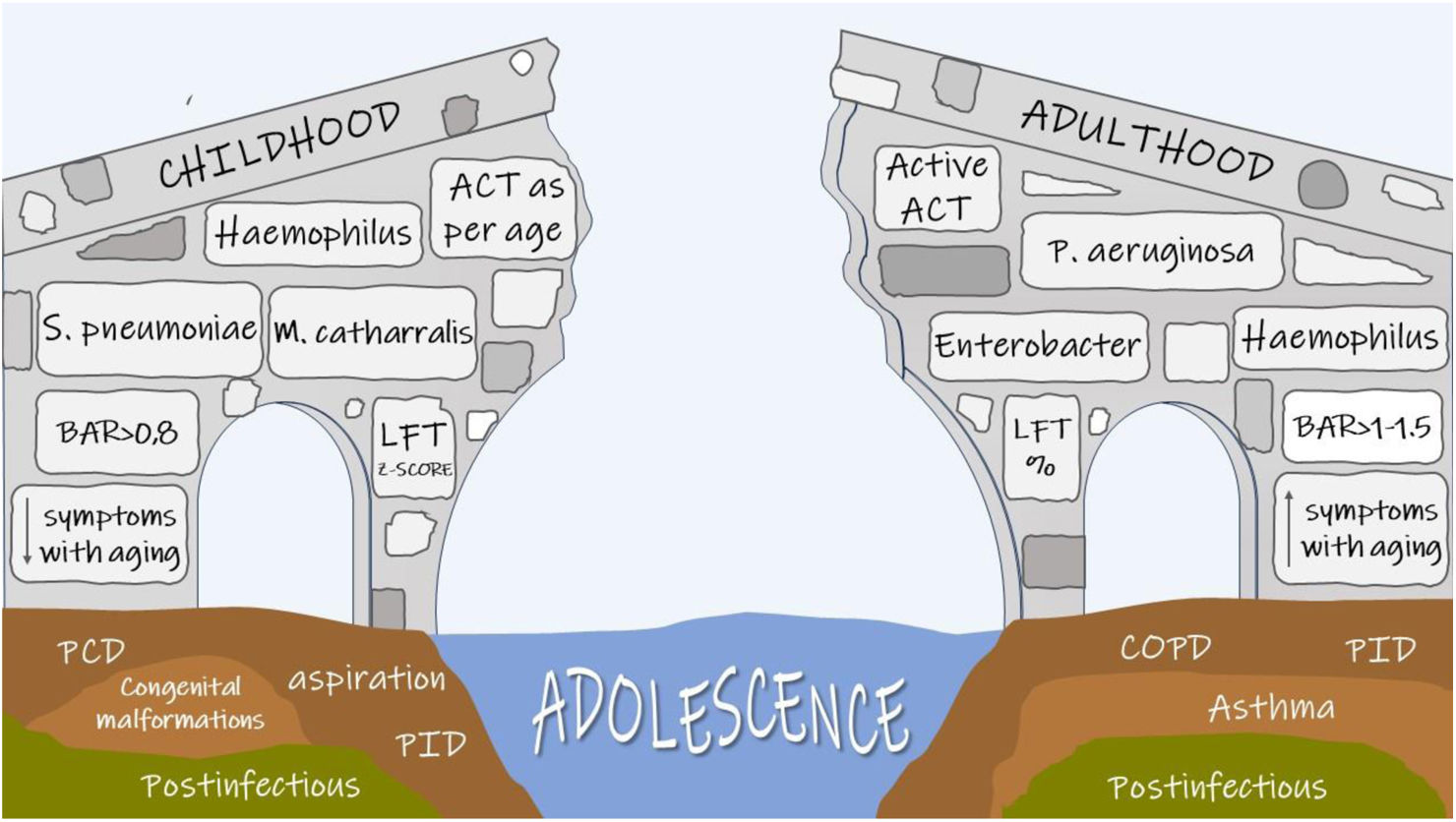
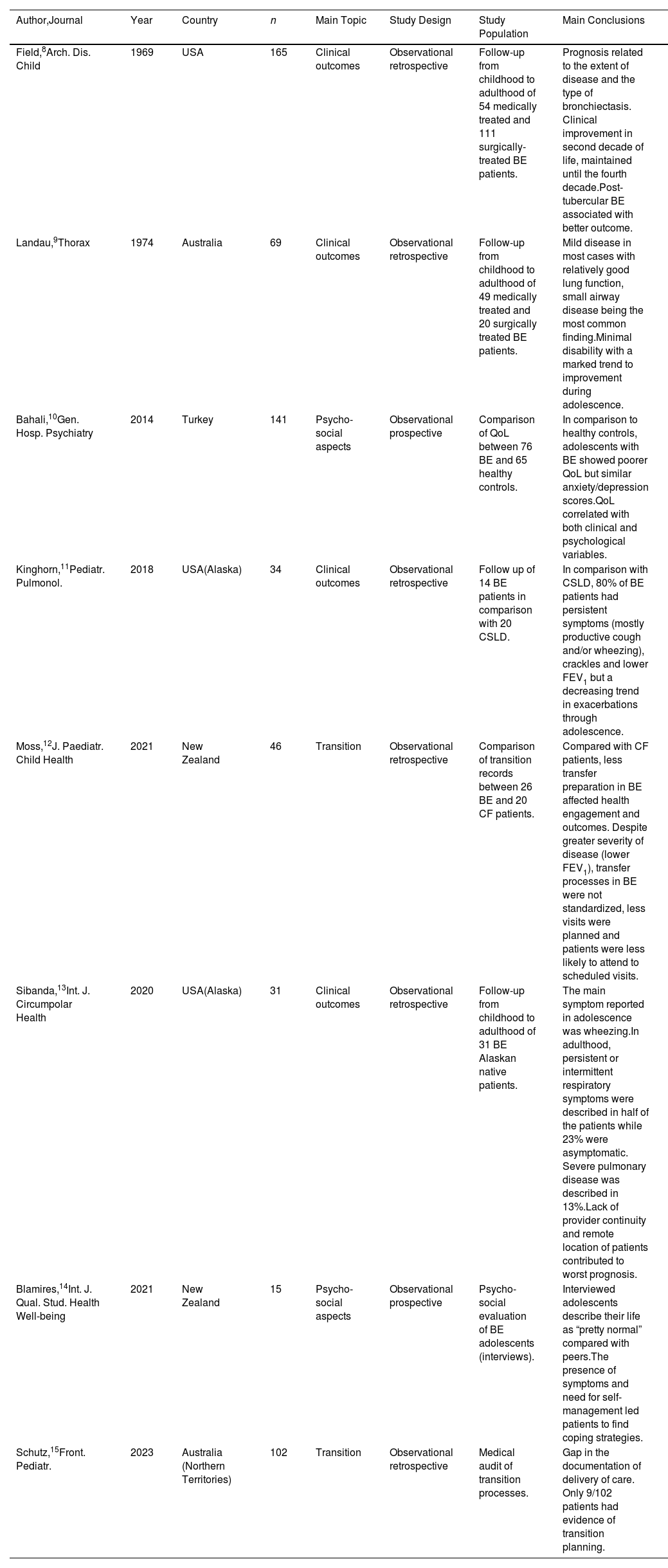
Paediatric and adult bronchiectasis patients have been addressed in the literature as two different populations due to several differences, but there is insufficient evidence to understand how and when disease characteristics really change along patients’ lifespan. This lack of knowledge is evident in all aspects of the transition: insufficient data is available about radiology, lung function, microbiology and treatment, and only limited information is currently available about changes in clinical presentation and psychosocial aspects. For instance, symptoms seem to improve during the third and fourth decades of life, a period sometimes referred to as the “honeymoon phase”. However, adolescents with bronchiectasis have poorer quality of life than healthy peers, suggesting, therefore, potential disease underestimation at this age.
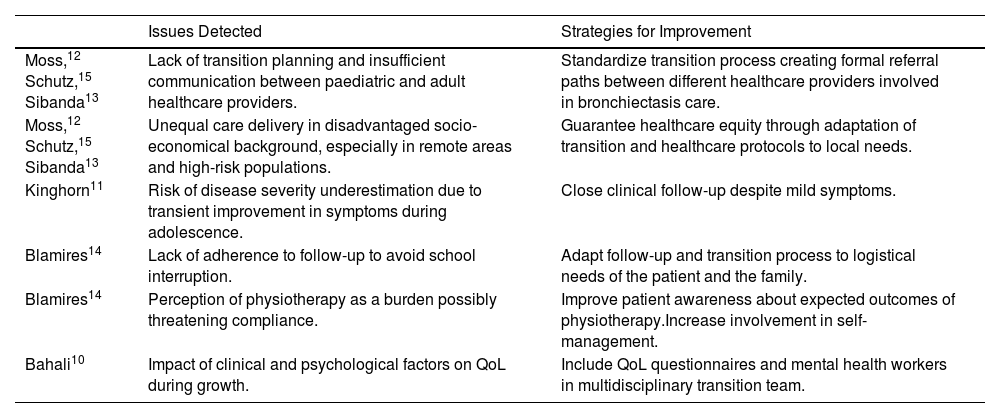
This scarcity of data most likely hinders the design of appropriate evidence-based transition protocols, ultimately limiting our ability to understand the factors driving disease progression and how to prevent it.
Nowadays it is crucial to raise awareness about this neglected aspect of bronchiectasis care, and fill this cultural and scientific gap by joining forces between pediatricians and adult physicians, to understand and stop disease progression and, lastly, to provide the best possible care to our patients in all phases of their lives.