Chronic obstructive pulmonary disease (COPD) is a public health problem due to its high prevalence (11% in the adult population in Spain), increasing incidence, and great social and economic impact. Despite this, it is underdiagnosed (and, therefore, undertreated) at a rate of around 80%. In this paper, a group of respiratory physicians specializing in COPD discuss 7 fundamental problems (“cardinal sins”) that contribute to this situation, with the explicit aim of proposing specific solutions that may help to improve this unfavorable state of affairs.
Chronic obstructive pulmonary disease (COPD) is a major public health problem due to its high prevalence (11% in the adult population in Spain), increasing incidence associated with population aging, and great social and economic impact.1–3 Despite this, around 80% of patients with COPD remain undiagnosed (and, therefore, untreated) and, in patients who have been diagnosed, the disease is often not confirmed by an appropriate diagnostic test (spirometry). As a result, treatment may not conform to national and international scientific recommendations.
Five years ago, Marin et al. published an excellent editorial in which they proposed 10 patient-focused “commandments” aimed at simplifying the treatment of COPD patients.4 In this document, a group of respiratory medicine specialists with experience in the diagnosis and treatment of COPD patients in Spain take a contrasting approach and identify and discuss the “7 cardinal sins” of disease management in Spain (not of treatment of the individual patient with COPD). The strategic objective of this discussion is to propose solutions that can be implemented to benefit COPD patients and improve the rational use of healthcare resources.
The 7 cardinal sins of COPD in SpainTable 1 lists the 7 cardinal sins discussed below and the actions proposed for correction. This “list” is the result of a discussion among the authors. Some readers may feel that other “sins” have been omitted from the list and/or others may disagree with those included. In any case, the authors hope to generate a discussion that will interest patients, healthcare professionals and administrators.
The 7 cardinal sins of COPD in Spain in 2021 and proposed solutions.
| Cardinal sin | Proposed solution |
|---|---|
| 1. Ephemeral public health plans | • Close monitoring of approved actions with public accountability |
| • Alliance with media on the importance of the disease and lack of implementation of approved programs | |
| 2. Lack of coordination in care | • Multidisciplinary units, led by Respiratory Medicine |
| • Integrated care programs | |
| • Advanced practice nursing in the COPD process | |
| • Day hospital for the management of severe patients | |
| 3. Diagnostic issues | • Expand the use of spirometry in Primary Care |
| • Train Primary Care staff in the correct use of spirometry | |
| • Improve knowledge of the disease in Primary Care and other medical specialties | |
| • Improve knowledge of the disease in nursing staff | |
| • Improve knowledge of the disease and its symptoms in the general population | |
| 4. Stigmatizing and stigmatized disease | • COPD is not just a disease caused by cigarette smoking |
| • It can begin in infancy and worsen through adolescence and early adulthood | |
| • It does respond to prevention and treatment | |
| • Early diagnosis and treatment should prevent disability in the elderly | |
| 5. Need for early action | • Perform spirometry studies in young people (<30 years) |
| • Identify respiratory health risk factors. | |
| 6. Clinical practice guideline adherence | • Reduce variability between CPGs. Simple messages avoiding discrepancies and proposals that generate confusion. |
| • Update guidelines regularly. | |
| • Disseminate guidelines appropriately. | |
| • Audit their impact on clinical practice. | |
| 7. Education of the population and patients | • Improve awareness in the general population: |
| • Informative campaigns in health media, social media and the general media | |
| • Identify a public figure who puts a face to COPD | |
| • Improve the education of COPD patients and their families/caregivers: | |
| • Simple and homogeneous educational material between the different care levels | |
| • Support for patient associations |
The Spanish National Health System (NHS) COPD Strategy, approved by the Interterritorial Council in 2009, cited various shortcomings in the organization of care, and established objectives and recommendations for improving the entire NHS.5 In its 2014 update, it became apparent that the degree of implementation of these proposals was “moderate”, and that while some activities had been started, their achievement was “limited and variable”.6 Furthermore, several autonomous regions in Spain have been generating their own documents, plans and care processes, but few end up being implemented. An expert report published in 2020 stated that3: “… so far, this [creating a structure for the management of COPD in Spain] has not been possible due to a lack of commitment and strategic initiatives coordinated and shared by political bodies and health authorities, managers, scientific societies, health professionals, associations and patient federations, media and related sectors”.
A real commitment is required to strengthen the National COPD Strategy and Regional Plans that includes the periodic evaluation of quantitative and qualitative indicators of their implementation and effectiveness. We also propose the creation of a Respiratory Health Plan3 that goes beyond COPD and addresses the importance of respiratory health in general, as the example of the COVID-19 pandemic has recently shown7; this would include funding for respiratory health prevention and promotion programs, early diagnosis and treatment of the most common respiratory diseases (including COPD), the understanding that forced spirometry is a non-invasive, inexpensive, reproducible test that provides information on respiratory health (with an impact on population aging),8 and a firm commitment to training, innovation and research in this area.
Lack of coordination in careSeveral healthcare areas and medical specialties are involved in the diagnosis and treatment of COPD, but there is no document defining their specific roles, how they should be coordinated and who is responsible for patient follow-up at each stage in the process.3 This results in undesirable variability in care, as shown by the COACH audit carried out in 63 randomly selected primary care centers in Spain9. This variability is also observed in the management of exacerbations. The AUDIPOC audit investigated the health outcomes of 5178 patients discharged following COPD exacerbation in 129 hospitals throughout Spain, and revealed huge differences between autonomous regions, hospitals and professionals in variables as important as mortality (ranging from 0% to 35%) and early hospital readmission (0%–62%) .10
COPD is a complex and heterogeneous disease that requires a personalized diagnostic and therapeutic approach, based on the “treatable traits” of each individual patient11; “standard” treatment for all patients is of no use.12 However, not all COPD patients are evaluated by specialists, so decisions affecting the most severe patients are not always made by the most experienced professionals. A recent study showed that, in primary care (PC), only 10% of patients diagnosed with COPD had been seen by a respiratory medicine specialist, while 24% of patients with a cardiac condition had been seen by a cardiologist.13
Improving care coordination is feasible. The creation of multidisciplinary functional units, led by respiratory medicine, facilitates the approach and organization of the entire COPD care process, from its prevention and early diagnosis, through complications and intercurrent exacerbations, to the final phase of life. Integrated COPD care programs are associated with lower costs and fewer readmissions.14 Day hospitals for the management and monitoring of severe patients play a key role in the care coordination process, by improving access to alternative emergency services, while avoiding contact with patients who attend the general emergency department and facilitating continuity of care.15 Finally, the role of advanced practice nursing is also fundamental in improving the continuity and quality of care provided to patients with COPD.16–18
Diagnostic issuesThe diagnosis of this disease is well described in clinical practice guidelines (CPG).1,19 Diagnosis is based on an assessment of respiratory symptoms, patient exposure to risk factors such as tobacco smoke and other harmful gases, and airflow limitation determined by post-bronchodilator spirometry. In principle, these patients should not be difficult to identify, but the evidence on the diagnosis of the disease in Spain suggests just the opposite. COPD is a disease with a high rate of diagnostic error, and this includes both underdiagnosis (almost 80% of COPD patients are undiagnosed) and, therefore, untreated20 and overdiagnosis (attribution of a COPD diagnosis to certain clinical presentations that are not actually COPD).
The impact of underdiagnosis is very serious, since failing to correctly diagnose patients with COPD in the early stages of their disease or detect it in advanced stages significantly reduces the possibility of mitigating disease progression and preventing exacerbations,21 thereby increasing the use of healthcare resources and healthcare costs associated with patient management.22 In Spain, more than 1.5 million people are currently unaware that they have COPD and are therefore not receiving the treatment and care they require.
Overdiagnosis is also a major public health problem. Studies, both national and international, carried out mainly in the PC setting, have shown that 30%–60% of patients classified as COPD or who have received treatment for COPD do not meet the diagnostic criteria established in clinical guidelines.23,24 A prospective study in Spain calculated the prevalence of COPD overdiagnosis in PC at 42.7%.25 This has substantial adverse consequences, in terms of both health (erroneous prescriptions of treatments and follow-ups) and costs (high use of resources by these patients).
Several factors help to explain these diagnostic problems. The most important include the misuse of spirometry in PC, either due to lack of access to spirometers in the centers (a situation that is increasingly rare), or due to underuse of the devices (lack of time and appropriately trained staff). Other problems include the non-specificity of symptoms or the belief that smoking is the only causative factor. For example, we now know that various situations during pregnancy, childhood and adolescence influence the development of COPD (not necessarily in relation to smoking) in adulthood.26 Finally, an additional factor that contributes to diagnostic errors is the lack of knowledge of this disease and its symptoms among the general population.3
Stigmatizing and stigmatized diseaseTraditionally, COPD has been understood (and explained, both professionally and socially) as a disease that is self-inflicted by smoking, appears in old age (in people in their sixties or seventies), and is progressive and irreversible.27–29 For this reason, it has historically been thought that smokers are to blame for their own condition, stigmatizing further both smokers and the disease and transferring responsibility to the patient.30,31 We are inclined to forget that smoking is more than a simple habit, or an activity associated with a lifestyle that is harmful to health, but reflects in most cases an addiction to nicotine, making it yet another item on the list of chronic addictive diseases that also includes drug addiction or alcoholism.32,33
This stigmatization of the smoker and the disease has several practical consequences. First, some smokers who experience coughing and dyspnea (possible COPD symptoms) interpret these symptoms (correctly) as the result of their smoking.34 However, because they do not want to feel ill due to their addiction, they tend to withdraw and cut down their daily and social activity in order to reduce exercise-associated respiratory distress, and delay seeking professional help until their symptoms already require urgent attention.34,35 Moreover, disease stigmatization affects not only the patient but also some healthcare professionals, who may adopt attitudes and practices that could contribute in part to late diagnosis, underdiagnosis or misdiagnosis, or to the idea that “little can be done”.36
This stigmatized view of COPD must change radically in the light of recent research showing that this perspective is clearly biased and incomplete. While smoking is a key (preventable) risk factor for COPD, it is not the only one. Today we know that other types of exposures (for example, to biomass smoke) and other events in the early stages of life (prematurity, low birth weight, repeat infections, poor nutrition) alter lung development and can facilitate the development of COPD in adulthood (even in young adults).37 Therefore, COPD is not related exclusively to smoking, nor does it appear exclusively in the later stages of life.26,38
To combat this stigmatization and its nihilistic consequences on therapy, COPD must be presented to the population and health professionals as a preventable and treatable disease that must be diagnosed at an early stage in order to improve patient outcomes.
Need for early actionAn early COPD diagnosis can impact positively on disease progression.39 This should prompt us to review our performance in this regard. The results of an audit carried out between 2014 and 2015 in 59 Spanish hospitals in patients diagnosed with COPD followed up in outpatient clinics, showed that the mean age of the patients was 70 years and that, in the case of patients under 55 years, medical professionals tended to adhere less to treatment guidelines.27 We must break away from the classic concept of COPD as a self-inflicted disease caused by smoking27 that affects older men and whose progression is prevented only by smoking cessation.28,29
The current definition of COPD based on forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) ratio is very specific,1,19 but today we know that this parameter is not very sensitive for detecting clinical (cough and chronic expectoration), physiological (decreased diffusing capacity for carbon monoxide [DLCO] or excessive drop in FEV1) and structural (areas of emphysema on computed tomography) lung changes which may precede spirometric obstruction.19 These changes have also been shown to adversely affect patient quality of life and, in some cases, to alter the course of the disease. This “spirometric-silent” period is currently a hotly debated scientific topic. Along these lines, the concept of “pre-COPD” has been recently proposed, in keeping with other medical specialties that have adopted the notion of a “pre-disease” state (e.g. pre-diabetes or pre-hypertension).41,42 In these therapeutic areas, the “pre-disease” stage does not mean that all patients will develop the pathology, but it identifies a certain at-risk population for closer and more individualized follow-up.
This new scenario for diagnosing and managing COPD at an earlier stage has been reinforced by observational studies in large patient cohorts. These have shown that at least 50% of patients diagnosed with COPD at 65 years of age already had lung function changes at 25 years of age.43 The natural history of the disease in this group of patients is different and closely related to lung development in the early years of life. However, this heterogeneity has not been taken into account in drug trials, and the possible differential effect of COPD treatment in this group of patients is unknown.
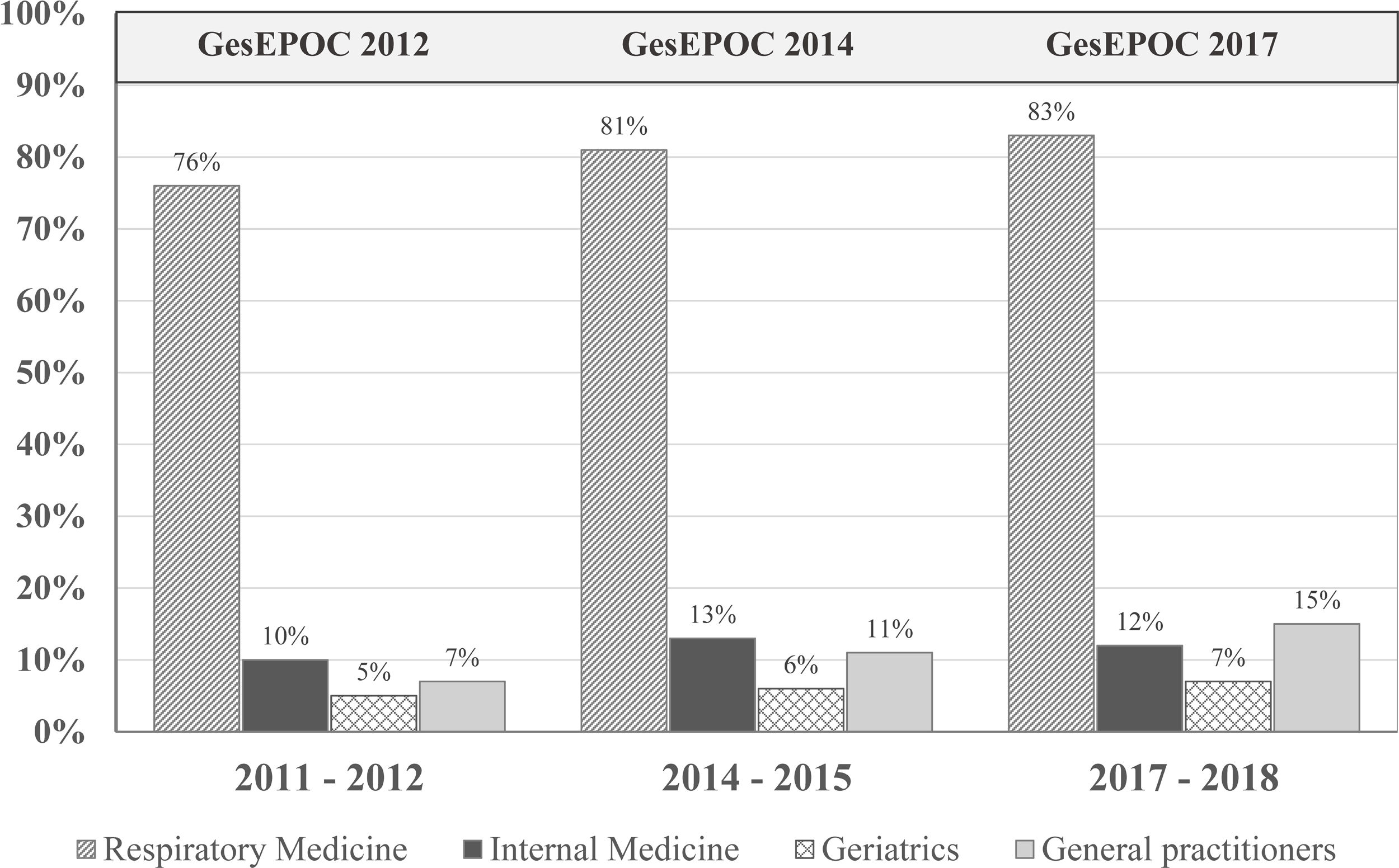
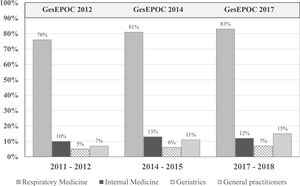
Clinical practice guideline adherenceThe main objective of CPGs is to reduce variability in clinical practice and improve effectiveness in disease management (in this case, COPD) through recommendations based on the best available scientific evidence (generally randomized clinical trials [RCTs] in large populations of carefully selected patients).44 A 2018 multicenter cross-sectional study in Spain found frequent deviations from the GOLD and Spanish COPD (GesEPOC) guidelines in clinical practice.45 In fact, several studies question their real usefulness.23,40,44 for several reasons. First, RCTs are necessary for assessing the efficacy and safety of any drug and are essential in the regulatory procedure, but their external validity is limited by the strict inclusion and exclusion criteria that determine the specific characteristics of the study population.46 Moreover, the applicability of the recommendations is not always taken into account when drawing up the CPG, although methods are now available to evaluate their implementation in the population, almost in real time.23 If a CPG fails to improve COPD management, its format and objectives should be reconsidered. Finally, the abundance of CPGs does not imply higher quality or practical utility. Variability between CPGs must be reduced, some confusing discrepancies must be eliminated, they must be continuously updated and appropriately disseminated, and their real clinical impact must be audited (Fig. 1).
Patients who have undergone spirometry to confirm the diagnosis of COPD in the last decade. The publication of the Spanish COPD guidelines (GesEPOC) and its updates has had little impact in improving the quality of the diagnosis in real life. Adapted from Izquierdo et al. (2021).23
Several studies in our setting show that the general public has a very poor understanding of COPD, including those at high risk of developing the disease.47,48 This lack of knowledge has also been found among their caregivers.49 Finally, we should mention that educational programs for patients and their family circle are implemented heterogeneously in different health areas.
This poor knowledge of the disease makes early diagnosis and treatment difficult,50 and hampers the implementation of self-care interventions in patients with COPD that may encourage smoking cessation, improve the inhalation technique, reduce anxiety and depression levels, and improve quality of life and use of health resources (unscheduled visits to emergency services, number of hospitalizations and length of hospital stays).51 The World Health Organization (WHO) global action plan for the prevention and control of non-communicable diseases (NCDs) 2013–2020 recommends empowering people with NCDs to better manage their own disease, and that education, incentives and tools for care and self-management are provided, including through the use of new information and communication technologies (ICT) such as eHealth or mHealth.52
Last but not least, a disease such as COPD, due to its prevalence, burden and social impact, should be well known to the general public, so that they can participate accordingly in its prevention, early detection and patient support.
ConclusionsThe 7 sins discussed here emerged from a debate among 7 respiratory medicine specialists with experience in COPD diagnosis, treatment and research. Our view, however, represents only part of the complex network of professionals, patients, caregivers, and administrators involved in the disease. Here, we discuss 7 elements we believe to be important, and propose specific initiatives to improve the current situation and, thereby, help to prevent and treat COPD earlier and better. In fact, a Lancet Commission of international experts is currently discussing whether such initiatives could eventually result in the “eradication” of COPD.53 The initiatives discussed here may help to achieve this goal at some point in the future.
Conflict of interestsDr JL Izquierdo reports having received compensation for Advisory Boards and consultation fees from: Astra Zeneca, Bayer, Boehringer ingelheim, Chiesi, GSK, Grifols, Menarini, Novartis, Orion, Pfizer, Sandoz Teva.
Dr C. Casanova reports having received fees and/or financial support for research projects of AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini y Novartis.
Dr. Bartolome R Celli reports having received compensation for Advisory Boards and consultation fees from: Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, Pulmonx, CHIESI, Menarini and Bayer. He does not have shares or interest in any company, neither does any member of my family. He has not received or had any relationship with tobacco money.
Dra S. Santos, does not present any conflict of interest with the present study.
Dr O. Sibila, does not present any conflict of interest with the present study.
Dra P. Sobradillo, does not present any conflict of interest with the present study.
Dr A. Agusti, reports having received fees and/or financial support for research projects of AZ, Chiesi, GSK, Menarini.
Acknowledgments and fundingThe authors would like to thank Dr. Susana Cañón and Dr. Blanca Piedrafita (Medical Statistics Consulting, S.L., Valencia, Spain) for their help in preparing and editing this article, in accordance with Good Publication Practice (GPP3) guidelines and thanks to the funding provided by AstraZeneca.