In the last fifty years, the role of small airways in chronic obstructive pulmonary disease has been thoroughly investigated owing to the availability of novel tools1 and technologies2 to assess different aspects of the multifaceted peripheral respiratory compartment once called “the lung silent zone”.3 Small airways with a diameter less than 2mm are a bridge between the central airways and the gas-exchange lung compartment. Under normal conditions, the contribution of small airways to the total airway resistance is modest.4 However, there is both direct and indirect evidence indicating that the contribution of this anatomical zone to the overall airway resistance, among others, becomes prominent in obstructive lung diseases, including asthma.5 Pathology has confirmed the presence of small airway abnormalities in asthma, irrespective of the degree of severity, from mild to moderate to very severe asthma.6,7 There are three key concepts to understanding the relevance of small airway dysfunction (SAD) in asthma: is SAD frequent in asthma? Is it related to the clinical outcomes and is it specific to any phenotype?
Is SAD frequent in asthma?In the last few decades, several methods have been developed to assess SAD; however, there is no single gold standard measurement. Indeed, each tool provides different information in the description/pathophysiology of SAD, from peripheral airway resistance, reactance, air trapping, heterogeneity of peripheral ventilation to imaging of the lung periphery.8 The studies conducted in this field consistently showed, by different techniques, the high prevalence of SAD across the entire spectrum asthma severity. The Assessment of Small Airways Involvement in Asthma (ATLANTIS) study recently specifically evaluated small airway impairment in patients with asthma by using all the available methods to assess SAD with different disease severities.9 This large multicentre study showed that small airway abnormalities can be identified by at least one of the tests in up to 90% of cases. This suggests that virtually all patients with asthma have small airway impairment.
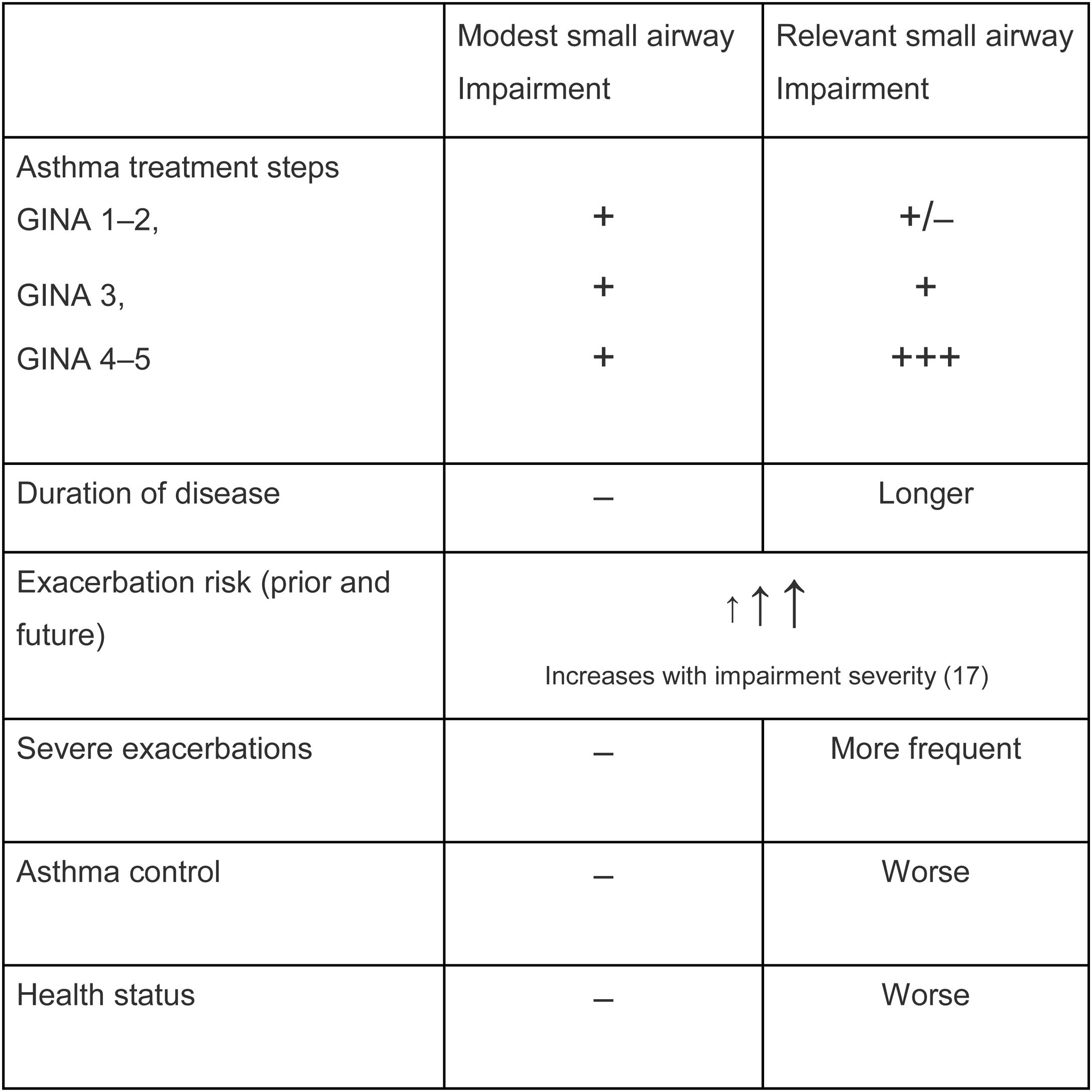
Is SAD related to clinical outcomes in asthma?From a clinical perspective, it is relevant to understand not only whether SAD is frequent, but more importantly, whether SAD affects clinical outcomes in asthma. SAD has been associated with important asthma clinical outcomes, such as dyspnoea,10 particularly in nocturnal asthma11,12 and exacerbations.13 Interestingly, several studies have shown that impairment in small airways, even in patients with normal lung function, is associated with a lack of asthma control. Such an association has been shown by means of functional parameters related to SAD, including impulse oscillometry and nitrogen washout test,14 as well as measurements of peripheral airway inflammation such as alveolar NO.15 These data clearly indicate a correlation between (low) asthma control and small airway impairment, even when FEV1 is still within the normal range. Additional analysis from the ATLANTIS study documented (as presented so far as scientific communications at international congresses) that the measurement of SAD in asthma is prospectively associated with the future risk of exacerbations.16 The Atlantis study, by assessing SAD with different tools, showed that the greater the impairment of small airways, the greater the severity of the clinical condition in terms of asthma control, exacerbation frequency and severity, quality of life, and treatment intensity.9 A composite score of SAD (ordinal score) obtained by IOS parameters predicted asthma exacerbations and asthma control, confirming, in a prospective fashion, the association between the dysfunction of small airways and these clinical outcomes.17 Notably, in a multivariate analysis, FEV1 was no longer a significant predictor of exacerbations when the ordinal score was included in the model.17 An overview of these relations are summarized in Table 1.
Small airways disease impairment and clinical outcomes.9,14,17
Phenotyping can allow personalized management and treatment. As such, the term “Trait” has been recently introduced, with “Treatable trait” indicating a clinical/marker characteristic for which a treatment is available. In severe asthma, some phenotypes with a poor response to treatment have documented small airway impairment. Findings from the Severe Asthma Research Network (SARP) showed that patients with severe asthma have prominent air trapping, detected as increased an RV/TLC ratio, rather than airflow obstruction defined as the FEV1/FVC ratio.18 When the trait “duration of the disease” was considered, higher lung function indices of air trapping correlated with small airway impairment in older asthma patients.19 A progressive and significant increase in residual volume over a 5 year follow up has been shown in asthmatic patients with a trait of fixed airflow obstruction compared with fully reversible airflow obstruction.20 Finally, smoking habits have deleterious effects and negative impacts on human health. In asthma, this trait reduces corticosteroid responsiveness, accelerates lung function decline, and increases the risk of poor disease control.21 Notably, cigarette smoking aggravates small airway abnormalities in patients with asthma.22
For all these reasons, we believe that evaluation of small airways is clinically important in the overall assessment of patients with asthma and that small airways are relevant targets for inhaled treatments. The small particle size of inhaled drugs has been shown to improve the deposition of delivered treatments throughout the entire bronchial tree, particularly in the peripheral compartment.23 Randomized control trials have shown that similar functional outcomes can be achieved with approximately half the dose of extrafine formulation as with non-extrafine formulations.24,25
ConclusionsGiven the relevance of small airways in the clinical outcome of asthma, the identification, by whatever means, of SAD could provide relevant information to optimize/personalize the management of asthma patients. Limiting the functional/inflammatory assessment of large airways (mainly FEV1) could preclude the identification of dysfunctions of the respiratory system that can be present very early in the evolution of the disease.
Conflict of interestsAP received grants for research from Chiesi, Astrazeneca, GSK, BI, Pfizer, Teva, Sanofi; Consulting fees from Chiesi, Astrazeneca, GSK, Mundipharma, Novartis, Boehringer Ingelheim, OM Pharma, Edmond, Sanofi, Avillion, IQVIA, Elpen Pharmaceuticals, TEVA, MSD, lecture fees form Chiesi, Astrazeneca, GSK, Mundipharma, Sanofi, MSD, Novartis, Zambon. MC reports personal fees from Alk-Abello; grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, grants, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees and non-financial support from Zambon, grants from University of Ferrara – Italy, outside the submitted work. FB and FA have no conflicts to declare.
The authors would like to thank Elisa Veratelli, University of Ferrara, for editorial support.