Pryce1 described pulmonary sequestration (PS) as a spectrum of abnormalities in which part of the lung, with or without bronchial communication, receives aberrant systemic arterial vascularization. The extralobar type is covered by the pleura itself and drains into the systemic veins, while the intralobar type shares the pleura with the rest of the lung and drains into the pulmonary veins; it accounts for 0.15%–6.4% of all congenital pulmonary malformations. Treatment has conventionally been surgical, although nowadays a less invasive option is available in the form of percutaneous treatment.2–4 Very few series of children with PS treated with embolization have been published, so we would like to report our experience in the percutaneous treatment of PS in a pediatric hospital in the Ecuadorian Andes.
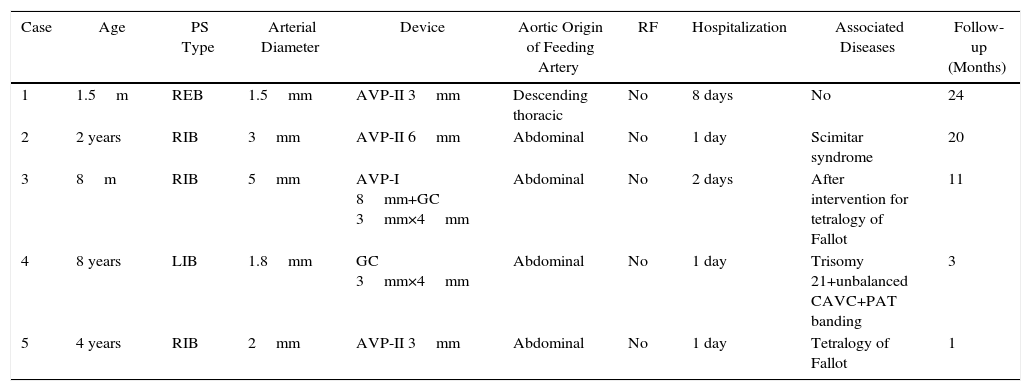
This was a cross-sectional study with consecutive sampling performed between March 2014 and April 2016. Data in common (Table 1): single artery feeding PS; vascular access via the femoral artery. No complications occurred, except in the first patient. Check-up with tomography was performed in patients 1, 2, and 3. Informed consent was obtained for all patients.
Common Data in Patients With Pulmonary Sequestration.
| Case | Age | PS Type | Arterial Diameter | Device | Aortic Origin of Feeding Artery | RF | Hospitalization | Associated Diseases | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5m | REB | 1.5mm | AVP-II 3mm | Descending thoracic | No | 8 days | No | 24 |
| 2 | 2 years | RIB | 3mm | AVP-II 6mm | Abdominal | No | 1 day | Scimitar syndrome | 20 |
| 3 | 8m | RIB | 5mm | AVP-I 8mm+GC 3mm×4mm | Abdominal | No | 2 days | After intervention for tetralogy of Fallot | 11 |
| 4 | 8 years | LIB | 1.8mm | GC 3mm×4mm | Abdominal | No | 1 day | Trisomy 21+unbalanced CAVC+PAT banding | 3 |
| 5 | 4 years | RIB | 2mm | AVP-II 3mm | Abdominal | No | 1 day | Tetralogy of Fallot | 1 |
AVP (I and II): Amplatzer™ vascular plug (I and II); CAVC: complete atrioventricular canal; GC: Gianturco coils; LIB: left intralobar base; m: months; REB: right extralobar base; RF: residual flow; RIB: right intralobar base; PAT: pulmonary artery trunk; PS: pulmonary sequestration.
In the first case, PS was suspected on radiograph, so we requested a tomography that confirmed a large PS. The procedure was complicated by reduced femoral pulse, treated with enoxaparin; the patient then developed systemic inflammatory response syndrome (SIRS) requiring intensive care. During follow-up, femoral pulses remained symmetric, pulmonary scintigraphy was normal, and the tomography showed full resolution of the PS.
In the other cases, PS was detected during catheterization. The second patient was catheterized due to suspected pulmonary hypertension on echocardiography. The third patient underwent catheterization due to poor progress after intervention for tetralogy of Fallot. Dilated bronchial arteries were detected and occluded with a 6mm Amplatzer™ vascular plug (AVP)-II, and an AVP-I was implanted in the vessel feeding the PS. Residual flow was observed, so a Gianturco coil (GC) was implanted, completing occlusion of the aberrant artery. The fourth patient underwent catheterization after developing cyanosis. The last patient had a diagnosis of tetralogy of Fallot and collateral vessels on echocardiography, so catheterization was indicated, during which the diagnosis was ruled out.
The essential component for diagnosing PS is angio-MRI, angio-tomography, or systemic artery angiogram of abnormal feeding of the pulmonary region. It is not yet clearly defined whether PS should be treated percutaneously or surgically, and the situation is even less clear if the patient is asymptomatic.3
Surgical treatment might require lobectomy, cause bleeding, infection or pneumothorax, and hospital stay is extended. Percutaneous treatment involves less risk of bleeding, is less incapacitating, and reduces length of hospital stay. However, complications, such as vascular events and SIRS, may occur, particularly in small children with large PS.
Several types of devices have been used for PS embolization2,4: we use AVP and GC because of their availability, safety and proven effectiveness in vascular occlusion, and their accessible, state-funded cost.
Patients were followed up with tomography 6 months after the procedure, and in all cases it was confirmed that the aberrant vessel had not rechanneled, the PS had resolved, and the residual lung had expanded. Resolution and expansion were particularly evident in the first patient, who had presented a very large PS; in this patient, the pulmonary scintigraphy also showed normal uptake in the right lung.
Although no significant differences have been identified between surgery and percutaneous intervention in terms of mortality, series with large numbers of patients undergoing surgery report 7–14 days of hospitalization, chest tube for 4 days, and lobectomy in most cases, particularly if the PS was intralobar.3,5 We did not make a comparative study of the 2 techniques, but these comorbidities are avoided with the use of percutaneous treatment.
To our knowledge, this is the second report of percutaneous treatment for PS in children in South America,4 and while endovascular was safe and effective in our series, it is still early to recommend it as an initial treatment choice, because more experience is required. However, thanks to the growing body of information about its effectiveness,2–4 it can be considered as a first treatment option in places where resources are limited in terms of intensive care beds or the availability of pediatric surgeons trained in the correction of congenital pulmonary disorders.
Please cite this article as: Ríos-Méndez RE, Andrade-Herrera JN, Aráuz-Martínez ME. Resultados a corto plazo del tratamiento percutáneo de secuestro pulmonar en hospital pediátrico ubicado en la región andina: serie de casos. Arch Bronconeumol. 2017;53:163–164.