The treatment of lung cancer has witnessed significant progress, leading to improved survival rates among patients. It is important to assess the individual contributions of non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) to overall lung-cancer incidence and mortality trends based population, especially sex difference.
MethodsWe analyzed lung cancer mortality based on subtype, gender, and calendar year. The Joinpoint software was used to identify any changes in incidence and trends in mortality.
ResultsIncidence and incidence-based mortality declined from 2001 to 2019 both NSCLC and SCLC annually. The most significant decrease occurred between 2016 and 2019 with annual percent change of 5.71%. From 2012 to 2016, the incidence-based mortality of SCLC in women changed by 2.7% in tandem with incidence decreased 2.84%. Remarkably, the incidence-based mortality for women declined notably by 5.23% between 2016 and 2019, even as the incidence showed a less extent of decreasing (-2.59%). The survival rate for women was 15.2% in 2001, 19.3% in 2016, it had increased to 21.3% in 2018 but similar trends not in men. The survival curve showed the change in survival outcomes over time among men and women (median overall survival: 13 vs 23months) receiving immunotherapy for SCLC.
ConclusionPopulation-level mortality from NSCLC and SCLC in the United States fell sharply from 2016 to 2019 as incidence deceased, and survival improved substantially. Our analysis suggests that approval for and use of immunotherapy may explain the mortality reduction observed during this period, with significant benefits especially for SCLC patient in women.
El tratamiento del cáncer de pulmón ha experimentado un progreso significativo, lo que ha mejorado las tasas de supervivencia entre los pacientes. Es importante evaluar las contribuciones individuales del cáncer de pulmón de células no pequeñas (NSCLC) y el cáncer de pulmón de células pequeñas (SCLC) a la incidencia general de cáncer de pulmón y las tendencias de mortalidad basadas en la población, especialmente la diferencia de sexo.
MétodosSe analizó la mortalidad por cáncer de pulmón en función del subtipo, el sexo y el año calendario. Se utilizó el software Joinpoint para identificar cualquier cambio en la incidencia y las tendencias en la mortalidad.
ResultadosLa incidencia y la mortalidad basada en la incidencia disminuyeron anualmente de 2001 a 2019, tanto el CPNM como el CPCP. La disminución más significativa se produjo entre 2016 y 2019 con un cambio porcentual anual de 5.71%. De 2012 a 2016, la mortalidad basada en la incidencia de CPCP en mujeres cambió en un 2,7% en conjunto con la incidencia disminuyó en un 2,84%. Sorprendentemente, la mortalidad basada en la incidencia para las mujeres disminuyó notablemente en un 5,23% entre 2016 y 2019, incluso cuando la incidencia mostró una menor disminución (-2,59%). La tasa de supervivencia para las mujeres fue del 15,2% en 2001, del 19,3% en 2016, había aumentado al 21,3% en 2018, pero no hubo tendencias similares en los hombres. La curva de supervivencia mostró el cambio en los resultados de supervivencia a lo largo del tiempo entre hombres y mujeres (mediana de supervivencia global: 13 vs. 23 meses) que recibieron inmunoterapia para el CPCP.
ConclusiónLa mortalidad a nivel de población por CPNM y CPCP en los Estados Unidos disminuyó drásticamente de 2016 a 2019 a medida que la incidencia falleció y la supervivencia mejoró sustancialmente. Nuestro análisis sugiere que la aprobación y el uso de la inmunoterapia pueden explicar la reducción de la mortalidad observada durante este período, con beneficios significativos especialmente para los pacientes con CPCP en mujeres.
Lung cancer is a major global public health concern, ranking second in newly diagnosed cancer cases at around 12-13%, and first in cancer-related deaths at approximately 21%.1 Lung cancer consists of a group of molecularly and histologically heterogeneous subtypes. Two major histologic subtypes are non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) which account for 80-85% and 15% of all cases of lung cancer, respectively in the United States.2,3 While incidence trends for these subtypes are well characterized, less is known about their respective mortality trends,4 especially the impact of treatment advancements on mortality. It is crucial to evaluate the mortality trends of various lung cancer subtypes since the potential implementation of lung cancer screening, along with treatment advance, is expected to have varying effects on future mortality from lung cancer differentially according to histologic subtype.5–7 Furthermore, it is important to calculate incidence-based mortality and survival rate separately for different genders due to the varying incidence of lung cancer. Actually, cancer survival rates vary greatly across different races due to factors such as socioeconomic status.8 Exploring lung cancer survival rates by race and ethnicity is crucial.
Over the years, significant advancements have been made in the treatment of NSCLC and SCLC with the introduction of targeted therapies and immunotherapy. One such example is the recommendation by the National Comprehensive Cancer Network (NCCN) in 2012 for all non-squamous NSCLC patients to undergo genetic testing for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements.9 Advancements in the identification and treatment of cancers that rely on these oncogenes, along with the approval of immunotherapy by the Food and Drug Administration (FDA) in 2015 have substantially improved outcomes of NSCLC.10 In comparison, the treatments for SCLC have shown limited data of mortality and improvements in efficacy. A series of clinical trials were conducted to investigate the use of programmed death-1 (PD-1) or programmed death ligand -1 (PD-L1) inhibitors in SCLC treatment. These trials included CheckMate 032, Keynote 604 and IMpower 133,11–14 revealing that PD-1/PD-L1 inhibitor, was a more effective treatment option for SCLC patients than chemotherapy. A more comprehensive assessment regarding the treatment advances and its impact on population-level mortality in SCLC is still lacking.
However, to understand lung-cancer incidence-based mortality trends and the effect of preventive interventions as compared with treatment interventions, it is important to assess the individual contributions of NSCLC and SCLC to overall incidence and mortality trends. In this study, the incidence -based mortality method was used to evaluate the mortality trends of NSCLC and SCLC in the U.S between 2001 and 2019. Our assessment considered the gender of patients in evaluating the contribution of incidence and cancer-specific survival to the observed mortality trends. This approach allowed us to gain insights into the gender-specific patterns and disparities in lung cancer incidence and outcomes. This study helps determine whether the observed changes in lung cancer mortality are primarily driven by changes in one type of lung cancer or if both types are equally affected.
Material and methodsStudy DataThe Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute is a reliable and comprehensive source of information on cancer incidence and mortality encompassing significant portion of the population in the United States. Our study population was based on SEER 17-registries database (SEER 17 Regs Research Data, Nov 2022[2000–2020 varying]) which was released April 2023.
To accurately classify the cancer subtypes, we implemented the Lewis et al. classification system, which utilizes the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) morphology codes for categorizing various cancer types. NSCLC subtype is defined using ICD-O-3 codes of 8003, 8004, 8012-8014, 8015, 8021, 8022, 8030, 8031-8033, 8034, 8035, 8046, 8050, 8051, 8052, 8070-8076, 8078, 8082, 8083, 8084, 8090, 8094, 8120, 8123, 8140, 8141, 8143-8145, 8147, 8190, 8200, 8201, 8211, 8240, 8241, 8243-8246, 8249, 8250-8255, 8260, 8290, 8310, 8320, 8323, 8333, 8401, 8430, 8440, 8470, 8471, 8480, 8481, 8490, 8503, 8507, 8525, 8550, 8560, 8562, 8570-8572, 8574, 8575 and 8576), while SCLC subtype is defined using ICD-0-3 codes of 8002 and 8041-8045. Since NSCLC was reliably identified starting from 2001 due to the evolving clinical practice, diagnostic methods, and classification,15 therefore we chose 2001 as the initial year for our analysis period to analyse accurately. Patients diagnosed with cancer through death certificate or autopsy were excluded from the study due to the unavailability of subtype information. Incidence and incidence-based mortality of lung cancer from 2001 through 2019 was calculated after accounting for COVID-19 pandemic and reporting delay.16
Statistical AnalysisSEER*Stat software (version 8.4.2) and Joinpoint software (version 5.0.1) from National Cancer Institute were used to download and analysis data. Lung cancer incidence and cancer-specific survival were assessed. The calculation involves dividing the number of cancer-related deaths recorded in the registry by the standard population. This method is used to assess the impact of screening and treatment on mortality trends for various cancer types.17,18
Our report presents data on incidence and incidence-based mortality categorized by year, gender, and subtype. We employed piecewise regression to characterize time trends in the age-standardized rates for each cancer subtype across different sexes. The Joinpoint program was used to select line segments for each curve, with the percentage associated with each line indicating the annual percentage change during the indicated range of years. The attached percentage represents the annual percentage change over the given time range. An asterisk (*) indicates that the annual percent change (APC) is statistically significantly different from zero (P<0.05).19 Lastly, we utilized the relative survival approach to estimate 2-year relative survival rates for lung cancer patients based on their sex, subtype and calendar year.18
Ethics StatementThis study was based on the SEER database and was conducted in accordance with the Declaration of Helsinki. Permission was obtained to access the research data of the SEER program, and informed consent was not necessary since the patients’ personal identities were not disclosed.
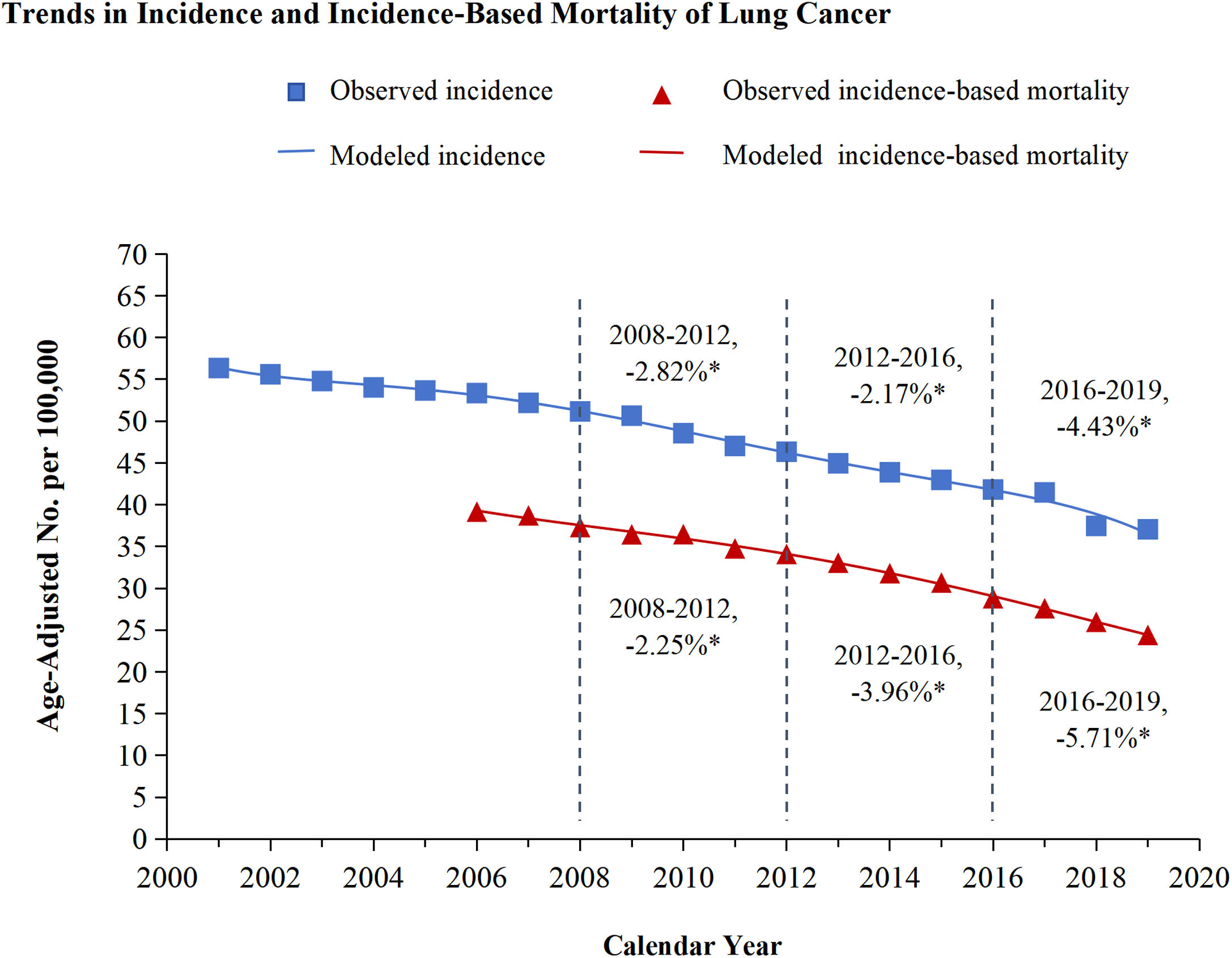
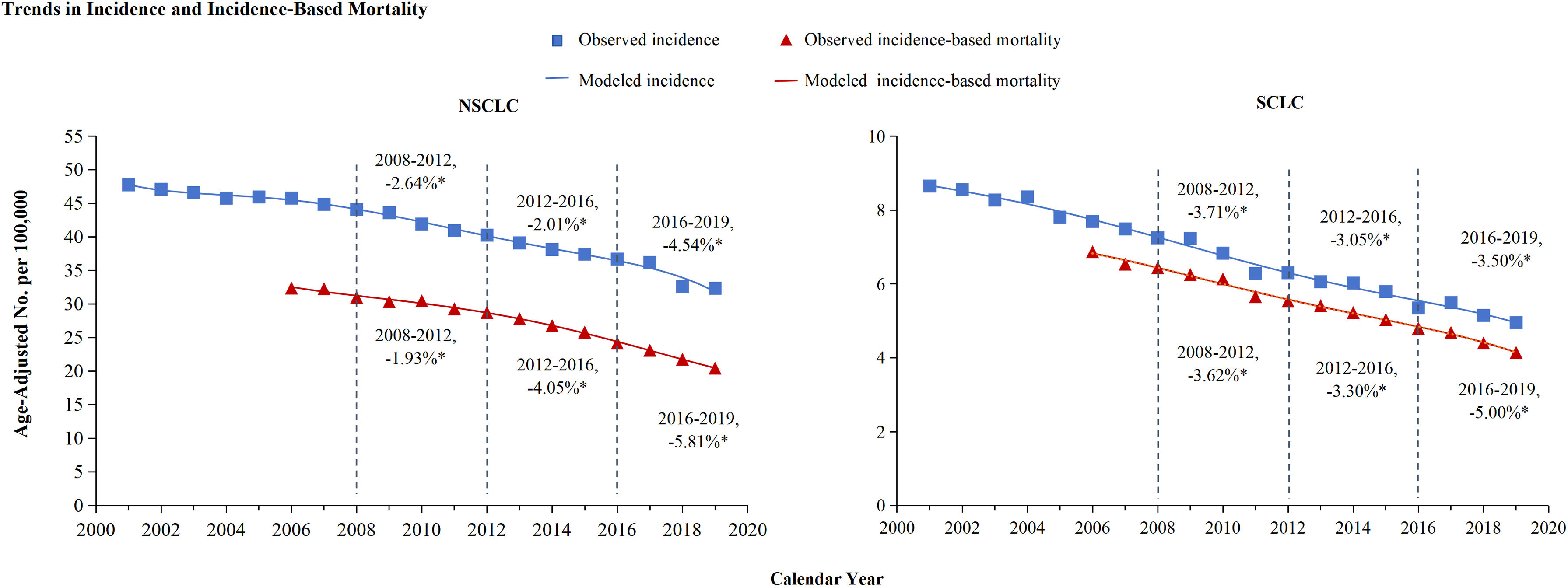
ResultsTrends in incidence and incidence-based mortality from Lung CancerThe incidence and incidence-based mortality of lung cancer from 2001 to 2019 were analyzed and exhibited in Figure 1. Lung cancer incidence declined each year from 2001 to 2019. From 2008 to 2012, the incidence declined by 2.82%, from 2012 to 2016, the incidence decreased by 2.17%, and from 2016 through 2019, the incidence APC faster decreased by 4.43% annually. Lung cancer incidence-based mortality also declined strongly from 2001 through 2019. From 2008 through 2012, the incidence-based mortality declined by 2.25%, then decreased more quickly by 3.96% annually from 2012 to 2016. The most significant decrease occurred between 2016 and 2019, with APC of 5.71% (Figure 1). Both NSCLC and SCLC incidence and incidence-based mortality decline each year from 2001 to 2019, and the rate of decline every three or four years is shown in Figure 2. Overall mortality from lung cancer has declined based on estimations of incidence, similar patterns were observed for histologic subtypes of lung cancer, especially for NSCLC and to a lesser extent for SCLC (NSCLC vs SCLC: -4.54% vs -3.50%). However, as targeted therapy progressed to immunotherapy, the incidence-based mortality for NSCLC and SCLC declined by comparable marginitude, with APC of NSCLC changing from 4.05% to 5.81% and SCLC from 3.30% to 5.00%.
Trends in incidence and incidence-based mortality of lung cancer
The figure shows the trends of modeled incidence for lung cancer (blue line) and the trends of modeled incidence-based mortality (red line). The observed incidence of lung cancer (blue squares) and the observed incidence-based mortality (red triangles) are shown. The attached percentage represents the annual percentage change over the given time range. Annual percentage changes with a significant difference from zero (P<0.05) are denoted by asterisks.
Trends in incidence and incidence-based mortality of non-small cell Lung cancer (NSCLC) and small cell lung cancer (SCLC).
The attached percentage represents the annual percentage change over the given time range. Annual percentage changes with a significant difference from zero (P<0.05 are denoted by asterisks. The two figures show the trends of modeled incidence (blue line) and modeled mortality based on incidence (red line) for NSCLC and SCLC respectively. Data are the observed incidence (blue squares) and the observed incidence-based mortality (red triangles).
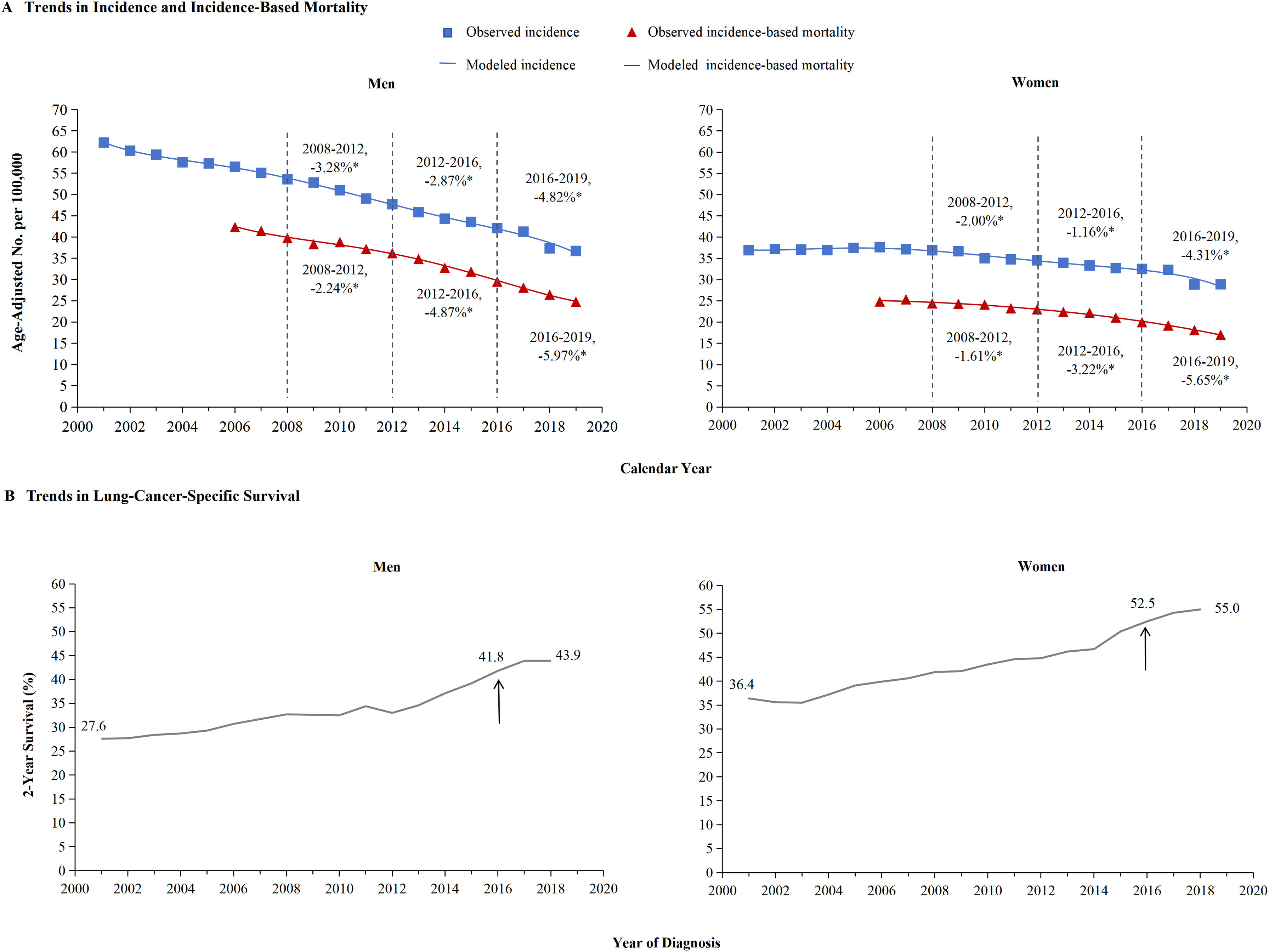
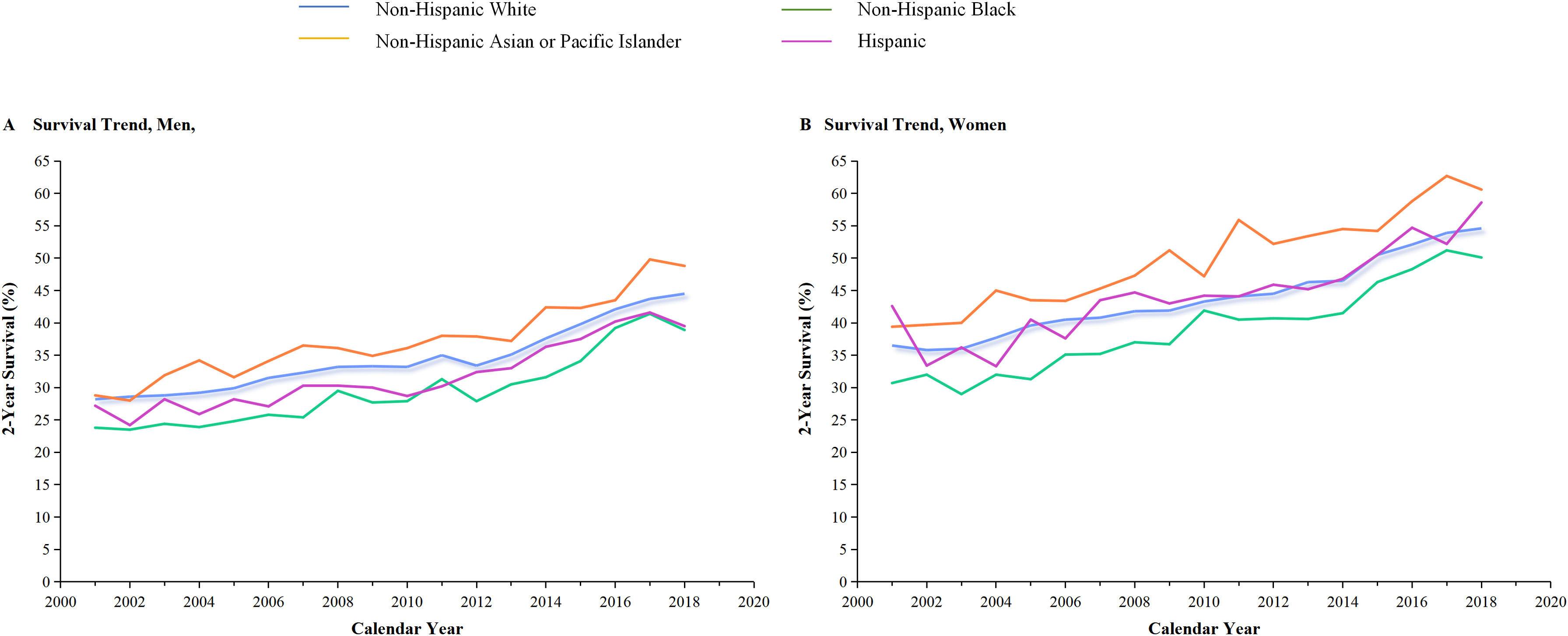
The incidence and incidence-based mortality of NSCLC from 2001 to 2019 were analyzed. Figure 3 provided a detailed breakdown of NSCLC incidence outcomes for men and women. The left portion showed that the incidence-based mortality of NSCLC in men gradually decreased by 2.24% from 2008 through 2012, followed by a significant decrease of 4.87% from 2012 to 2016. Between 2016 and 2019, the incidence-based mortality in men dropped by 5.97%. The 2-year relative survival rate for men with NSCLC saw a significant increase from 27.6% in 2001 to 43.9% in 2019 (Figure 3B). The occurrence of NSCLC among women remained steady from 2001 through 2008 and then began to drop by 2.00% from 2008 through 2012, followed by a further 1.16% decrease from 2012 through 2016. However, incidence-based mortality showed a gradual reduction, decreasing by 1.61% from 2008 through 2012, 3.22% from 2012 through 2016, and 5.65% from 2016 through 2019 (Figure 3A). Similar pattern based on estimations of incidence and incidence-based mortality was observed for men and women, especially for men and to a lesser extent for women. This decline in incidence-based mortality surpassed the decline in incidence, indicating the effectiveness of targeted therapies and immunotherapies, particularly in men. In 2013, the FDA approved first-line EGFR-targeted therapy, which resulted in a significant decrease in incidence-based mortality among both men and women compared to previous years. The panel displayed the generation of immunotherapy in 2016, as evidenced by a change in slope. Over the years, there has been a marked improvement in NSCLC incidence, particularly among women (2016-2019). The result for cancer–specific survival of NSCLC was exhibited according to race and ethnic group among men (Panel A) and women (Panel B). In 2001, the 2-year survival rate for women was 36.4%, which increased to 55% by 2019 (as shown in Figure 3B). Additionally, improvement in cancer-specific survival was observed in all races and ethnic groups (Figure 5).
The incidence, incidence-based mortality, and survival trends of non-small cell lung cancer (NSCLC) among men and women.
Panel A shows incidence (blue line) and incidence-based mortality (red line) for the NSCLC histologic subtype among men and women. The observed incidence of lung cancer (blue squares) and the observed incidence-based mortality (red triangles) are shown. Panel B displays the 2-year cancer-specific survival among men and women, categorized by diagnosis year. The 2-year survival rate for men has significantly improved from 27.6% in 2001 to 43.9% in 2019, while for women from 36.4% to 55%.
Survival trends of non-small cell lung cancer (NSCLC) among men and women categorized by race and ethnicity.
The 2-year cancer-specific survival for NSCLC subtypes is presented by race and ethnicity in Panels A and B for men and women, respectively. Among all species, non-Hispanic Asian or Pacific Islander had the highest two-year survival, while Non-Hispanic Black had the lowest.
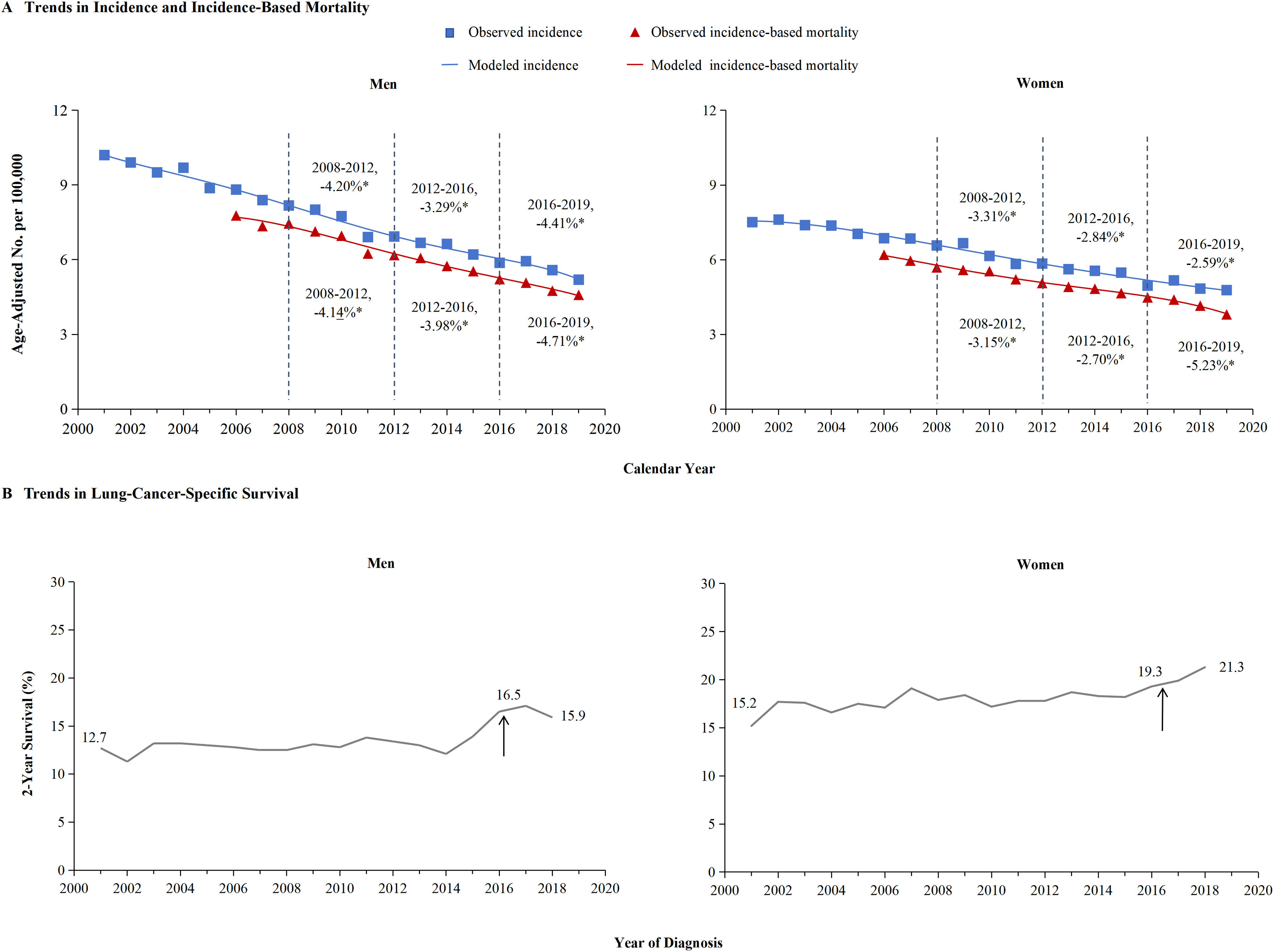
Figure 4 provided a detailed breakdown of SCLC incidence and incidence-based mortality for men and women. Different trends were observed for incidence and incidence-based mortality. The decline in mortality from SCLC in men is more likely to benefit from the decline in incidence may contributed to early detection or public health interventions, especially from 2012 through 2016. The left portion of Figure 4A showed that incidence of SCLC in men declined each year from 2001 to 2019. From 2008 to 2012, the incidence declined by 4.20%, from 2012 to 2016, the incidence decreased by 3.29%, and from 2016 through 2019, the incidence rate faster decreased by 4.41% annually. Meanwhile, the incidence-based mortality of SCLC in men gradually decreased by 4.14% from 2008 through 2012, followed by a significant decrease of 3.98% from 2012 to 2016. Between 2016 and 2019, the incidence-based mortality in men dropped by 4.71%. The left portion of Figure 4B indicates 2-year survival rate for men with SCLC did not significantly increase from 2016 to 2019, 16.5% in 2016 to 15.9% in 2018.
The incidence, incidence-based mortality and survival trends of small cell lung cancer (SCLC) among men and women.
The attached percentage represents the annual percentage change over the given time range. Annual percentage changes with a significant difference from zero (P<0.05) are denoted by asterisks. Panel A shows incidence (blue line) and incidence-based mortality (red line) for the SCLC histologic subtype among men and women. The observed incidence of lung cancer (blue squares) and the observed incidence-based mortality (red triangles) are shown. Panel B displays the 2-year survival rate among men and women, categorized by diagnosis year. The 2-year survival for men has significantly improved from 12.7% in 2001 to 15.9% in 2019, while women from 15.2% to 21.3%.
Remarkably, in women with SCLC, the incidence-based mortality declined in a pattern different to that of the incidence among men. The incidence of SCLC in women declined each year from 2001 to 2019. From 2008 to 2012, the incidence declined by 3.31% annually. From 2012 to 2016, the incidence-based mortality in women changed by 2.7% in tandem with incidence decreased 2.84%. However, the incidence-based mortality declined notably by 5.23% between 2016 and 2019, even as the incidence of SCLC in women showed lesser decreasing in the period of 2016-2019(-2.59%) compared to 2012-2016 (2.84%) (Figure 4A).
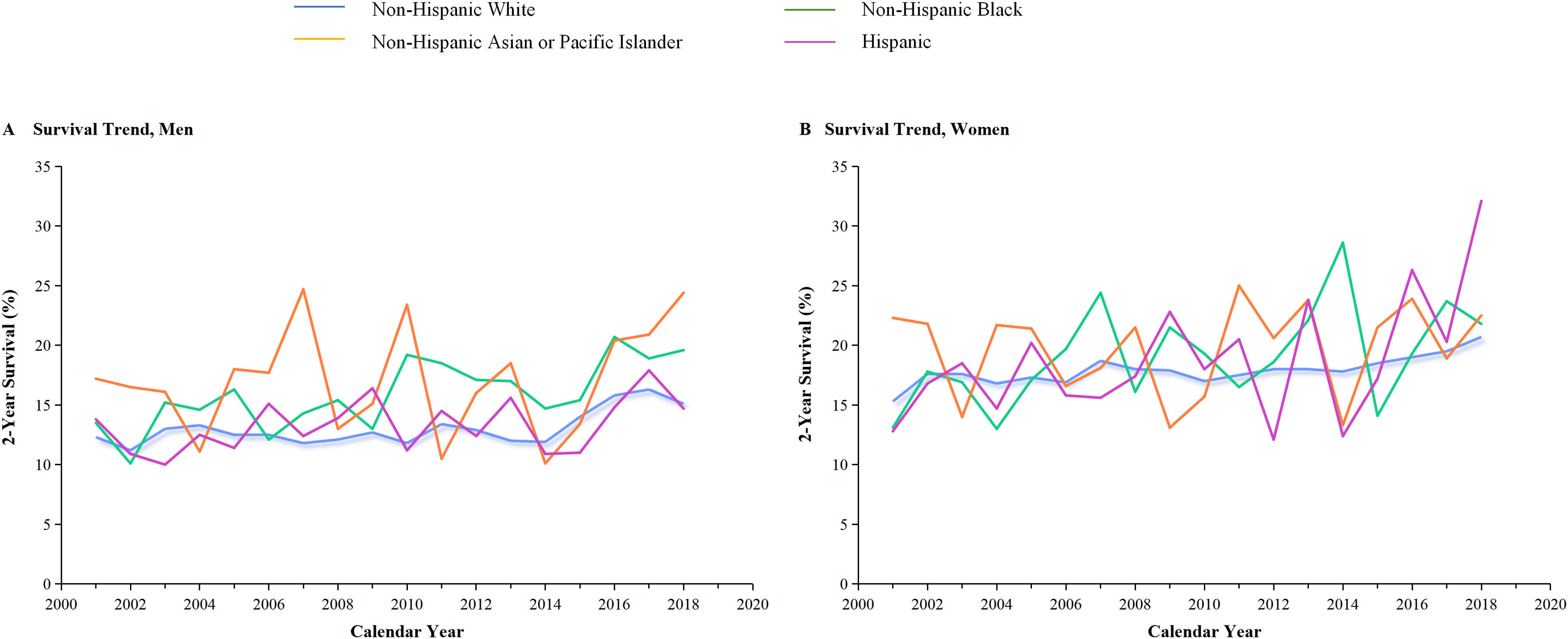
We further analyzed the survival rate of SCLC in women. The right portion of Figure 4B demonstrates significant improvements in the 2-year survival rates for women over the years. In 2001, the survival rate for women was 15.2%, 19.3% in 2016, but by 2018, it shad strongly increased to 21.3%,while 16.5% in 2016 decreased to 15.9% in 2018 for men. This data suggests that there has been an improvement in the 2-year survival rate for women significant increased compared to men over year, especially from 2016 through 2019. The decline in SCLC mortality in women is not just due to a decline in incidence, but more likely to be due to advances in treatment. The effectiveness of immunotherapies for women with SCLC was indicated by the result as evidenced by a change in slope. In a single-center cohorts, the survival curve showed the change in survival outcomes over time among men and women (median overall survival, 23 vs 13months, P=0.088) which verify the role of gender in SCLC patient received immunotherapy (Supplementary Figure 1). In addition, improvements in cancer-specific survival rates were observed to vary across racial and ethnic groups (Figure 6). The result for 2-year specific survival for the SCLC in gender was different according to race and ethnic group among men (Panel A) and women (Panel B). Among all species, non-Hispanic Asian or Pacific Islander in men had the highest survival. Non-Hispanic white had the lowest in women.
Survival trends of small cell lung cancer (SCLC) among men and women categorized by race and ethnicity.
The 2-year lung cancer-specific survival rate for SCLC subtypes is presented by race and ethnicity in Panels A and B for men and women, respectively. It is evident in the panel that the two-year survival rate for Hispanic women has shown a significant increase from 2014 to 2019.
In this study, we describe trends in mortality among patients with various subtypes of lung cancer, taking into account the changes in incidence and survival patterns. Overall incidence and incidence-based mortality from lung cancer has declined. Different patterns were observed for histologic subtypes of lung cancer based on estimations of incidence-based mortality. Specifically, we found a rapid decline in incidence-based mortality from SCLC in women during the period from 2016 through 2019, shortly after immunotherapy was recommended and commercial use of FDA-approved immunotherapy. And there was no significant change in mortality for SCLC in men, even though the incidence in men has fallen more dramatically. For NSCLC, we found steadily declining mortality from 2016 through 2019 both in men and women.
The Effect of Advances in NSCLC Treatment on Population MortalityThe overall mortality of lung cancer has decreased. Both declining incidence and improving survival have driven the decline in mortality from NSCLC and SCLC. Our results update previous analyses of trends in lung-cancer incidence according to histologic subtypes.20 Over the past decade, there have been significant changes in the approach to treating NSCLC. Specifically, the mortality of NSCLC experienced a rapid decline between 2012 and 2016, which was further accelerated in 2013 when molecular alterations in EGFR and ALK were detected and targeted therapies were introduced and approved by the FDA. The mortality declines faster in 2016-2019 contributed to immunotherapy advance. The difference in mortality reduction of NSCLC between men and women was not significant, but improvement in cancer-specific survival was observed. Clinical trials have investigated the benefits of immunotherapy in patients with NSCLC including CheckMate 017, IMpower150 trial, OAK trial and CheckMate 057 trials.21–23 The KEYNOTE-010 study show that pembrolizumab provided long-term overall survival(OS) benefit and durable responses among patients receiving 2-years of treatment for previously treated, PD-L1?expressing advanced NSCLC patients.24 And atezolizumab is also indicated for previously treated NSCLC and improves survival.21 In addition, nivolumab outperformed docetaxel in terms of OS, response rate, and progression-free survival (PFS) among patients with advanced regardless of PD-L1 expression level.25 The IMpower150 trial explored the role of immunotherapy combined other therapy, the results has shown improved OS and PFS of combination of atezolizumab, bevacizumab, carboplatin and paclitaxel than any of them alone. These findings strongly suggest that the immunotherapy improvement in cancer-specific survival rates.
Sex differences in NSCLC mortality were described in our study, with significantly improved survival for both men and women. Previous studies have corroborated our results, genetic testing and targeted therapies may benefit more men with NSCLC,20 and immune checkpoint inhibitors can improve OS for patients with NSCLC, but the magnitude of benefit is sex-dependent.26 Survival was significantly better in men than women received chemoimmunotherapy.27 Results of the phase 3 trial KEYNOTE-024 have been presented, comparing pembrolizumab with standard chemotherapy in patients with NSCLC. The subgroup analyses reveal a remarkable increased relative benefit with immunotherapy for male patients, while only a minimal benefit in female patients.28,29 These data seem to suggest that patients’ sex predicts different relative benefit achievable from immunotherapies. Meanwhile, 2-year lung cancer-specific survival has improved for NSCLC from all racial and ethnic groups among men and women. The survival benefit for patients with NSCLC treated with targeted therapy and immunotherapy has been shown in clinical trials, but our study highlights their possible effect at the population level among men and women.
The Effect of Advances in SCLC Treatment on Population MortalityIn our research, we noted that SCLC incidence rates were lower than NSCLC incidence rates and that the decline in incidence was more pronounced in men than in women. This discrepancy can be attributed to the higher proportion and relative risk of smoking in SCLC cases compared to the NSCLC cohort. The decline in lung cancer incidence rates was more significant in men, likely due to differences in smoking prevalence between genders.6,7,17 According to our research, mortality decreased significantly from 2016 to 2019 when immunotherapy applications were introduced shortly after the relevant clinical trials were concluded compared to 2012-2016. The trend of 2012-2016 may be attributed to SCLC has no clear treatable driver target, while the trend of 2016-2019 may be influenced by clinical trials.11,12,14,30–32 CheckMate 032 was started in 2012, have focused on immunotherapy as a viable treatment option for SCLC patients who test positive for PD-L1. The final results of this trial revealed that the PD-1 inhibitor (nivolumab) was considerably more effective than chemotherapy. This groundbreaking discovery has opened up new possibilities for immunotherapy in SCLC.33 Subcequently, results from IMpower 133 clinical trial suggest that he long-term survival rate of atezolizumab for extensive-stage small cell lung cancer (ES-SCLC) was higher than that of placebo, thereby strongly confirming the benefit of immunotherapy.34,35 While immunotherapy isn’t effective for all patients, it has been successful in generating significant and long-lasting responses in about 20% of them. These treatments have played a vital role in reducing morbidity-based mortality rates over the last decade. Nivolumab became available in the U.S. in 2015 and was officially approved by the FDA in 2018 for use as a first-line treatment for SCLC. Additionally, the 2018 NCCN Guidelines recommend the use of nivolumab in combination with ipilimumab as a first-line treatment option for SCLC patients with PD-L1≥1%.36 This indicates that the advance of immunotherapy for SCLC had a considerable impact on overall progress.
To the best of our knowledge, our findings provide the first evidence of a significant difference in immunotherapy advance among sex for SCLC based population mortality. Remarkably, survival rate of female patients with SCLC significantly improved between 2016 and 2019. The mortality faster decreased in SCLC among women than men. The mortality for SCLC has decreased may due to the faster decline in SCLC mortality among women. Results of IMpower133 updated in 2020.35 The subgroup analyses show an impressive increased relative benefit with immunotherapy in female patients and only a minimal benefit in male patients (OS, HR: 0.59, 95% CI (0.42-0.82) vs 0.94, 95% CI(0.68-1.10)). These data in IMpower 133 presented seem to suggest that patients'sex predicts different relative benefit achievable from immunotherapy, regardless of tumor expression levels of PD-L1. There is an association between sex and survival in SCLC. To our knowledge, it has several potential implications for clinical practice and future research. The first consideration is that patient's sex should be considered when assessing the risk and benefit of treatment strategies, as it is an important factor in predicting the potential benefits of immune checkpoint inhibitors. Further research is required to validate this pattern and ascertain the influence of gender on immunotherapy. The second implication of our work is that researchers designing new immunotherapy studies should ensure that women and men participate equally in clinical trials.
In all, our study has yielded significant findings. Firstly, we have produced estimates on the incidence, mortality and survival patterns of various lung cancer subtypes across the entire United States population, with emphasis on female patients with SCLC. Secondly, our estimates are founded on reliable cancer registry data from the SEER program, which guarantees precise and comprehensive documentation of all newly diagnosed cancer cases within the registry. Prior study indicated that mortality from lung cancer may be lower than currently reported and incidence-based mortality could be captured more accurately from SEER database, which was more approach than with National Center for Health Statistics death certificate data.20 While there is no data on drugs specific to immunotherapy available for analysis in the SEER database, studying the impact of treatment progression on mortality using large population-based data holds significance. Finally, it is worth mentioning that our results surpass those from individual centers in terms of representativeness. While clinical trials may not encompass adequately diverse samples of older, seriously ill, and low-income patients, our study has taken a more inclusive approach. Hence, our findings provide a more comprehensive depiction of the effect of advances in lung cancer treatment on population incidence and mortality.
ConclusionsPopulation-level mortality from NSCLC and SCLC in the United States fell sharply from 2016 to 2019 as incidence decreased, and survival after diagnosis improved substantially. Our analysis suggests that approvals for and use of immunotherapy may explain the mortality reduction observed during this period, especially patient with SCLC in women benefits significantly.
Ethics approval and consentThe data based on the SEER database was conducted in accordance with the Declaration of Helsinki. Permission was obtained to access the research data files of the SEER program. Informed consent was not necessary as the patients’ personal identities were not disclosed. The data from single-center cohort was approved by the SDTHEC-2023003129. Written informed consent was obtained from all patients.
FundingThis work was funded by the National Natural Science Foundation of China (82172676, 82373217), the Natural Science Foundation of Shandong (ZR2021YQ52, ZR2020LZL016), and the Young Elite Scientist Sponsorship Program by Cast (no. YESS20210137).
Author contributionsMY and YWQ analyzed the data. JGS was a major contributor in collecting the data. MY and DCW wrote the manuscript. MW and DWC contributed to revising the manuscript. DCW and JMY were major contributors in supervising the manuscript. All authors read and approved the final manuscript.
Consent for publicationNot applicable.
Conflicts of interestThere are no conflicts of interest to declare.
Availability of data and materialsThe data underlying this article were accessed from SEER database [URL: https://seer.cancer.gov/]. The derived data generated from SEER database and single-center cohort in this research are available from the corresponding author on reasonable request.
We are grateful to the work of SEER database and American National CancerDatabase.