Vaccines are a fundamental public health tool, particularly effective in preventing respiratory infections. Their importance is amplified in patients with chronic respiratory diseases, who are more susceptible to acute infections such as influenza, pneumococcus, COVID-19, respiratory syncytial virus (RSV), pertussis, and herpes zoster. These individuals face an increased risk of severe infections, exacerbations, hospitalizations, and mortality. Vaccination against influenza, pneumococcus, and SARS-CoV-2 significantly reduces these adverse outcomes. Evidence also supports the use of vaccines against RSV, pertussis, and herpes zoster in this population. In specific cases, tailored immunization strategies are warranted. The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) strongly advocates for systematic vaccination in patients with chronic respiratory diseases. This document provides clear and up-to-date recommendations based on the available evidence to support clinical practice and standardize vaccination strategies. These recommendations aim to reduce complications, improve quality of life, and enhance public health outcomes in this vulnerable population.
Vaccines are among the most effective public health tools for the prevention of numerous infectious diseases, helping to reduce both morbidity and mortality.1 Their impact is particularly significant in the prevention of respiratory infections, which are a major cause of hospitalization and death worldwide.1,2 As a prevention strategy, mass vaccination campaigns go beyond protecting vaccinated individuals; they also contribute to herd immunity by reducing the spread of pathogens and protecting the most vulnerable groups, such as children, the elderly, and people with comorbidities.1,3
Vaccines are particularly relevant for people with chronic respiratory diseases. Patients with these conditions already have compromised respiratory systems, significantly increasing their vulnerability to severe respiratory infections such as influenza, pneumonia, and respiratory syncytial virus (RSV) infection.4–8 These infections not only worsen lung function but can also trigger exacerbations that may require hospitalization and intensive treatment, or even result in death.9,10
In this context, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) reaffirms its commitment to promoting vaccination as an essential strategy to protect respiratory patients and improve their prognosis. Vaccines against influenza, pneumococcus, and other respiratory pathogens help prevent serious complications in this population, improving their quality of life and reducing the risk of adverse respiratory events.11,12
This document aims to serve as a clinical practice guide for healthcare professionals. Its use will help ensure that patients with chronic respiratory diseases receive adequate protection against preventable respiratory infections. The methodology followed for its development is detailed in Supplementary Material 1.
QuestionsQuestion 1. Do patients with chronic respiratory diseases have a higher risk of contracting infections caused by influenza, Streptococcus pneumoniae, COVID-19, Bordetella pertussis, RSV, or herpes zoster?In the case of influenza, concurrent chronic lung diseases such as asthma or chronic obstructive pulmonary disease (COPD) are associated with a higher risk of hospitalization, need for ventilatory support, and admission to the intensive care unit (ICU.14 Specifically, asthma increases pneumonia risk, while COPD is associated with a higher likelihood of requiring ventilatory support.14
Patients with such chronic respiratory diseases also have a higher risk of developing community-acquired pneumonia (CAP) and invasive pneumococcal disease (IPD); the risk of CAP increases between 1.3 and 13.5 times, while that of IPD is elevated by 1.3–16.8 times.10 CAP risk varies by underlying condition and age, with individuals aged ≥65 years with COPD being particularly vulnerable.10 Asthma is also a significant risk factor for IPD, with an odds ratio (OR) of 2.44 (95% confidence interval [CI] 2.02–2.96).15 Notably, children (aged 0–17) with asthma are more susceptible to IPD than adults aged ≥18 years,15 even when immunized with the 7-, 10-, and/or 13-valent pneumococcal vaccines.16 A Spanish study also identified chronic respiratory diseases as strong predictors of pneumococcal pneumonia (hazard ratio [HR] 2.66; 95% CI 2.47–2.86).17
Since the emergence of the SARS-CoV-2 infection, various chronic diseases have been associated with a higher risk of unfavorable outcomes.18 Respiratory disorders, including interstitial lung diseases and pulmonary hypertension, are associated with more severe SARS-CoV-2 infection and higher mortality.18 Moreover, COPD patients show higher COVID-19 incidence (2.51%; 95% CI 2.33–2.68) than the general population over 40 years (1.16%; 95% CI 1.14–1.18; p<0.001); they also suffer worse prognosis, including increased hospitalizations and mortality.19 Asthma is also a risk factor, especially for severe COVID-19 cases.18 Among children under 2 years hospitalized for COVID-19, chronic lung disease is associated with increased severity (relative risk [RR] 2.2; 95% CI 1.1–4.3).20
A U.S. retrospective study assessed the incidence of pertussis in patients with asthma or COPD.21 Among adolescents over 11 years and adults with asthma, pertussis incidence was 0.27 per 1000 person-years (95% CI 0.27–0.29), with an RR of 3.96 (95% CI 3.81–4.12) compared to controls; the highest rates were among adults aged 19 to 64 years (RR 4.06, 95% CI 3.86–4.27).21 For COPD, the RR was more than twice that of controls (RR 2.53, 95% CI 2.40–2.68), and was also highest in those aged 19 to 64 years (RR 3.59, 95% CI 3.35–3.84).21
For RSV infection, COPD significantly increases hospitalization risk in adults over 50, with admission rates 10 times higher than in those without the condition.22 Additional evidence links COPD and chronic respiratory failure to increased ICU admission risk from severe RSV disease.23 In asthma patients, RSV-related hospitalization is 7–8 times higher across all ages compared to those without asthma.22 Among children with chronic respiratory disease, RSV also poses a risk factor for poor outcomes (OR 3.64; 95% CI 1.31–10.09).24
Herpes zoster risk is also elevated in those with asthma (RR 1.24; 95% CI 1.16–1.31)25 and COPD (RR 1.41; 95% CI 1.28–1.55).24 Moreover, COPD exacerbations tend to increase around episodes of herpes zoster, with a notable rise observed in the month preceding diagnosis.26
This evidence highlights the impact of these infections in patients with chronic respiratory diseases and reinforces the need for effective prevention strategies.
Question 2. Is vaccination against influenza, S. pneumoniae, COVID-19, B. pertussis, RSV, and herpes zoster effective in reducing severe exacerbations and/or mortality in patients with COPD?InfluenzaInfluenza vaccination is recommended for patients with COPD (Recommendation A)Influenza infection is a major trigger of COPD exacerbations.27 Mortality risk is increased in patients over 75 years old and residents of long-term care facilities, those with cardiac comorbidities, and those receiving home oxygen therapy.28 In COPD patients, mucus hypersecretion and impaired mucociliary function create a favorable environment for virus proliferation.4 As a result, the virus is responsible for up to 28% of COPD exacerbations,29 playing a key role in the morbidity and mortality of these patients.11
A systematic review and meta-analysis on the effects of influenza vaccination in COPD patients showed a significant reduction in exacerbations and a trend toward decreased hospitalization.30 Subgroup analysis revealed that this reduction was especially prominent in patients with a forced expiratory volume in one second (FEV1) below 50% of predicted.30
In patients who have suffered a myocardial infarction or have high-risk coronary disease, influenza vaccination has been shown to reduce both all-cause and cardiovascular-related risk of death.31 Given that COPD and coronary artery disease share smoking history as a common risk factor, preventing cardiovascular events through influenza vaccination is particularly important for these patients.32
S. pneumoniaePneumococcal vaccination is recommended for patients with COPD (Recommendation B)COPD and lower respiratory tract infections (LRTIs) are major global causes of death. Meanwhile, approximately 55% of all LRTI-related deaths are attributable to pneumococcal pneumonia.33,34 COPD patients who develop CAP experience greater severity, more frequent ICU admission, and significantly higher 30- and 90-day mortality compared to those without COPD.6,35 Among individuals with COPD, S. pneumoniae is the most commonly identified microorganism.36
COPD patients are at greater risk of hospitalization due to CAP and IPD compared to those with other chronic lung diseases.10,37 Among adults aged 40 and older hospitalized for CAP, those with COPD are overrepresented, being 18 times more likely to develop CAP than those without.5 This increased risk is attributable to impaired airway defense mechanisms, use of inhaled corticosteroids, and smoking.10,37
The CAPITA study, which involved over 84,000 adults aged 65 and older, demonstrated that the 13-valent pneumococcal conjugate vaccine (PCV13) is effective in preventing pneumococcal pneumonia and IPD.12 Meanwhile, a study in China showed that the 23-valent pneumococcal polysaccharide vaccine (PPSV23) significantly reduced the risk of acute exacerbations (54%), pneumonia (53%), and related hospitalizations (46%) in COPD patients.38
Both the Centers for Disease Control and Prevention (CDC) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend pneumococcal vaccination for COPD patients.13,39 However, most available studies are older and used polysaccharide vaccines. More recent studies have evaluated currently available conjugate vaccines.
The latest CDC recommendations extend the indication for PCVs to adults aged 50 and older.40 A recent study demonstrated that the 20-valent PCV (PCV20) is cost-effective in adults over 65 years.41
COVID-19SARS-CoV-2 vaccination is recommended for patients with COPD (Recommendation B)SARS-CoV-2 vaccines have been highly effective in reducing emergency visits, hospitalizations, and ICU admissions, both in the general population and in individuals with chronic respiratory diseases.42,43 Although most studies have focused on the general population, observational studies suggest a protective effect on mortality among COPD patients, despite notable biases.44,45 The observed reduction in exacerbations in some populations may result from decreased virus circulation, underscoring the indirect benefits of immunization.46
Both the GOLD guidelines and the CDC recommend following national COVID-19 vaccination protocols to ensure protection and prevent complications in COPD patients.13,46
RSVRSV vaccination is recommended for patients with COPD (Recommendation B)RSV causes respiratory illnesses ranging from mild upper respiratory tract infections to severe bronchiolitis and pneumonia.32 A four-year prospective cohort study showed RSV infection occurring in 3–7% of healthy older adults and 4–10% of high-risk adults.8 Immunosenescence may contribute to increased disease severity and trigger exacerbations of chronic respiratory conditions in older adults.47
CDC and GOLD guidelines recommend RSV vaccination for adults aged 60 and older,13,48 especially those with chronic comorbidities, including cardiac or respiratory diseases, due to the higher risk of RSV infection in these patients.48
B. pertussisPertussis vaccination is recommended for patients with COPD (Recommendation B)Pertussis is a contagious respiratory infection characterized by persistent coughing.32 COPD may increase both the risk and severity of these infections. In a retrospective cohort study in the UK, COPD patients had a 2.32 times higher risk of contracting pertussis, resulting in greater healthcare use and higher medical costs.49
Herpes zosterHerpes zoster vaccination is recommended for patients with COPD (Recommendation B)COPD increases the risk of herpes zoster infection and its complications at any age, with a higher risk in patients using inhaled corticosteroids.50,51 A meta-analysis found a 41% increased risk of herpes zoster in COPD patients compared to healthy controls.25 The GOLD guidelines and the CDC recommend herpes zoster vaccination for individuals aged 50 and older, although healthcare systems should consider including all adults with COPD in their vaccination programs.13
Question 3. Is vaccination against influenza, S. pneumoniae, COVID-19, B. pertussis, RSV, and herpes zoster effective in reducing severe exacerbations and/or mortality in people with asthma?InfluenzaInfluenza vaccination is recommended in patients with asthma (Recommendation A)In asthma patients, antiviral immunity in the respiratory tract is impaired by chronic airway inflammation and type 2 immune response, increasing their susceptibility to severe influenza and associated bacterial infections.52 Mechanisms of this increased predisposition include weakened innate immune, deficient type 1 helper T-cell responses, and reduced interferon-alpha production by plasmacytoid dendritic cells in response to influenza.53 Meanwhile, influenza infections can trigger severe asthma exacerbations, often requiring hospitalization.54
Influenza vaccination has been shown to significantly reduce exacerbations in asthma patients.55 Therefore, annual influenza vaccination is recommended for both adults and children with moderate to severe asthma.56
The live attenuated intranasal vaccine, indicated for children aged 2 years and older, carries warnings and precautions in cases of severe asthma or active wheezing. However, some recent studies have reported the use of this vaccine in asthmatic children without adverse effects.57,58 Nevertheless, other studies have reported wheezing (but not hospitalizations) in days following intranasal vaccine administration. Consequently, the CDC does not recommend administering this vaccine to children aged 2–4 years with an exacerbation in the previous 12 months, and advises caution for other asthmatic children.59
S. pneumoniaePneumococcal vaccination is recommended in patients with asthma (Recommendation B)Patients with chronic respiratory diseases, including asthma, have a higher risk of developing CAP and IPD compared to individuals without these comorbidities.10
While specific studies in asthma are limited, clinical guidelines recommend pneumococcal vaccination in people with severe asthma.39,56 The GINA guidelines advise reviewing local pneumococcal vaccination policies for asthmatic children.60 In Spain, the Spanish Association of Pediatrics (AEP) recommends expanding coverage with the PCV20 in severe asthma, with preference over the PPSV23, in children previously vaccinated with PCV13 or PCV15.61
COVID-19SARS-CoV-2 vaccination is recommended in patients with asthma (Recommendation B)SARS-CoV-2 vaccination in asthma patients is critically important, especially due to low immunization rates in this population.62 While most reports have focused on the general population, observational studies suggest that vaccines reduce mortality in asthma patients, despite some biases.46,47
Both the GINA guidelines and the CDC agree on following national vaccination recommendations to ensure adequate protection for individuals with asthma against SARS-CoV-2.46,56 In children, the AEP recommends vaccinating those with severe asthma from 6 months of age.61
RSVRSV vaccination is recommended in patients with asthma (Recommendation B)In children, RSV infection causes LRTIs such as bronchiolitis and pneumonia. In older adults, such infections can exacerbate pre-existing respiratory conditions, including asthma.63
Current RSV vaccines are effective in preventing RSV-related acute respiratory infections. Both CDC and GINA guidelines recommend administration in individuals aged 60 and older,13,48 especially in those with coexisting conditions, including asthma.48 In children, there are currently no approved RSV vaccines. However, passive immunization with monoclonal antibodies, such as nirsevimab, is available to prevent RSV infection in children under 2 years at risk of complications such as severe asthma.61
B. pertussisPertussis vaccination is recommended in patients with asthma (Recommendation B)The prevalence of asthma in patients hospitalized for pertussis is 26% for adults and 44% for adolescents.64 Moreover, pertussis is associated with greater asthma severity, resulting in more frequent symptoms and worse lung function.65 Pertussis may also contribute to more severe asthma exacerbations, causing prolonged coughing and subsequent sleep disturbances.66
Vaccination against pertussis reduces the risk of severe disease, although evidence on its efficacy and safety in preventing asthma exacerbations is limited. Our recommendation aligns with guidance for patients with other chronic respiratory diseases.56,64
Herpes zosterHerpes zoster vaccination is recommended in patients with asthma (Recommendation B)Asthma increases the risk of herpes zoster and related complications, especially in patients using inhaled corticosteroids.50,67 A meta-analysis found a 24% increased risk of developing herpes zoster in asthma patients compared to healthy individuals.25 Therefore, herpes zoster vaccination may be indicated in patients with asthma.
Question 4. Is vaccination against influenza, S. pneumoniae, COVID-19, B. pertussis, RSV, and herpes zoster effective in reducing severe exacerbations and/or mortality in patients with lung cancer?InfluenzaInfluenza vaccination is recommended in patients with lung cancer (Recommendation B)Patients with cancer, particularly of the lung, are at increased risk of influenza-related morbidity and mortality.7 Malignant neoplasm onset has been linked to higher influenza prevalence in the year prior to detection,68 and cumulative viral exposure may increase lung cancer risk.69
Antiviral treatment or influenza vaccination may reduce hospitalizations and mortality in cancer patients.70 The CDC and the American Society of Clinical Oncology (ASCO) recommend annual influenza vaccination for cancer patients of all ages.71
S. pneumoniaePneumococcal vaccination is recommended in patients with lung cancer (Recommendation B)Infections are a common cause of lung cancer progression, linked to reduced treatment response, worse prognosis, and increased mortality.72 Research has explored the role and involvement of S. pneumoniae in lung cancer progression and carcinogenesis.73,74 The ASCO recommends pneumococcal vaccination for all adults with cancer.71
COVID-19SARS-CoV-2 vaccination is recommended in patients with lung cancer (Recommendation B)COVID-19 vaccines effectively reduce emergency visits, hospitalizations, and ICU admissions.42,43 Vaccination is essential in cancer patients to reduce severe complications.
Lung cancer patients can safely mount an adequate immune response post-vaccination, regardless of cancer treatment.75 According to the latest CDC recommendations for immunocompromised individuals, the vaccine is advised at all ages—an indication supported by the ASCO.71
RSVRSV vaccination is recommended in patients with lung cancer (Recommendation B)The CDC and ASCO recommend RSV vaccination for adults over 60, especially those with chronic lung disease or immunosuppression, to prevent severe illness.48 These vaccines should be included in immunization programs, prioritizing high-risk and particularly vulnerable groups such as lung cancer patients. They can be administered simultaneously with other seasonal immunizations.71
B. pertussisPertussis vaccination is recommended in patients with lung cancer (Recommendation B)Immunity against tetanus, diphtheria, and pertussis—covered by the Tdap vaccine—declines with age, and this decline may accelerate after cancer treatment.76 Cancer patients should receive the Tdap vaccine if not previously vaccinated in adulthood.
Herpes zosterHerpes zoster vaccination is recommended in patients with lung cancer (Recommendation B)Herpes zoster incidence is particularly high during the first two years after cancer diagnosis, particularly for hematologic malignancies like multiple myeloma.77 Patients under 50 have a higher risk compared to older adults. Complications such as postherpetic neuralgia negatively affect quality of life. For this reason, the ASCO recommends herpes zoster vaccination in adults over 19 with lung cancer.71
Question 5. Is vaccination against influenza, S. pneumoniae, COVID-19, B. pertussis, RSV, and herpes zoster effective in reducing severe exacerbations and/or mortality in patients undergoing lung transplantation?InfluenzaInfluenza vaccination is recommended in lung transplant recipients (Recommendation B)Respiratory viruses, including influenza, cause significant morbidity and mortality in lung transplant patients.78 There is also a strong epidemiological link between influenza and subsequent pneumococcal pneumonia.79 The American Society of Transplantation (AST) recommends the inactivated influenza vaccination before and after transplant.80
A cohort study in 357 patients found that antibody responses to the influenza vaccine were stronger in those vaccinated before transplantation and in long-term survivors compared to those vaccinated shortly after the transplant.81
In immunosuppressed pediatric lung transplant recipients, the intranasal live-attenuated vaccine is contraindicated; the quadrivalent inactivated vaccine is preferred. Close contacts and caregivers should also be vaccinated.58
S. pneumoniaePneumococcal vaccination is recommended in lung transplant recipients (Recommendation C)Lung transplant recipients have an increased risk of pneumococcal and other LRTIs due to immunosuppression.82 Those who develop IPD or pneumonia have significantly higher mortality rates. Humoral immune status is also impaired post-transplant.83
The recommended strategy for solid organ transplant candidates is to administer as many vaccines as possible before transplantation, including pneumococcal vaccines.80 Monitoring humoral immune responses to pneumococcus may help identify high-risk transplant recipients.84,85
CDC guidelines for immunocompromised patients recommend a single dose of PCV15, PCV20, or PCV21 if previously unvaccinated. If PCV15 is used, PPSV23 should be given at least 8 weeks later. Previously vaccinated patients (with PCV13 or PPSV23) should wait at least one year before receiving newer conjugate vaccines.86
In immunosuppressed children, the AEP Vaccine Advisory Committee recommends completing vaccination with PCV20. If unavailable, PPSV23 may be used in children over 2 years of age. The minimum interval between PCV20 and any earlier conjugate vaccine is 8 weeks; however, if PCV20 is given after PCV13 or PCV15 and a dose of PPSV23, a 12-month gap from the PPSV23 dose is required.61
COVID-19SARS-CoV-2 vaccination is recommended in lung transplant recipients (Recommendation B)Lung transplant patients are at higher risk for severe SARS-CoV-2 infection due to immunosuppression.87
In children, the AEP recommends COVID-19 vaccination for immunosuppressed children from 6 months of age and their close contacts.61
RSVRSV vaccination is recommended in lung transplant recipients (Recommendation B)Lung transplant patients are highly vulnerable to severe RSV infections. The CDC advises RSV vaccination for adults over 60 who are immunosuppressed or have chronic lung disease to prevent serious illness.21
In children under 2 years of age who have received a lung transplant, nirsevimab is recommended at the start of RSV season.61
B. pertussisPertussis vaccination is recommended in lung transplant recipients (Recommendation B)Immunosuppressed patients are more vulnerable to severe infections such as pertussis, which may cause serious respiratory complications. AST guidelines recommend Tdap vaccination before and after transplantation.80
Herpes zosterHerpes zoster vaccination is recommended in lung transplant recipients (Recommendation C)The inactivated zoster vaccine is recommended both pre- and post-transplant, as it does not contain live viruses and is therefore safe for immunocompromised individuals. It should be administered during a stable post-transplant period to prevent serious complications.76 The live-attenuated vaccine is only advised pre-transplant.80
Question 6. Is vaccination against influenza, S. pneumoniae, COVID-19, B. pertussis, RSV, and herpes zoster effective in reducing severe exacerbations and/or mortality in patients with other chronic respiratory diseases?InfluenzaInfluenza vaccination is recommended in patients with other chronic respiratory diseases (Recommendation B)Influenza vaccination is crucial for individuals at higher risk of severe influenza-related illness and complications, and to prevent outpatient, emergency, and hospital visits.88 This includes people aged 50 or older, and adults and children with chronic pulmonary or cardiovascular diseases.88
These recommendations also appear in clinical guidelines for pulmonary hypertension and bronchiectasis.89,90 In bronchiectasis patients, influenza and pneumococcal vaccinations reduce exacerbations.91 Although data are limited in interstitial lung disease, historical evidence suggests benefits in reducing mortality and hospitalization risk.92
The AEP also recommends vaccinating children with chronic respiratory diseases from 6 months of age, along with their close contacts.61
S. pneumoniaePneumococcal vaccination is recommended in patients with other chronic respiratory diseases (Recommendation B)Clinical guidelines suggest that individuals with chronic respiratory diseases are at increased risk for serious pneumococcal infections.39 The pneumococcal vaccine reduces the risk of infection and complications. Combination with the influenza vaccination reduces the occurrence of both overall66 and hospital-related exacerbations.9
For children, the AEP59 and CDC93 recommend extending pneumococcal vaccine coverage using PCV20 if available, and PPSV23 otherwise. In immunosuppressed patients receiving PPSV23, a repeat dose is recommended after 5 years.
COVID-19SARS-CoV-2 vaccination is recommended in patients with other chronic respiratory diseases (Recommendation B)Vaccines effectively prevent COVID-19 hospitalizations and respiratory failure in chronic respiratory patients.94 Vaccination is strongly encouraged in this group to reduce the risk of serious complications and improve outcomes.
In children, the AEP recommends COVID-19 vaccination from 6 months of age in those with chronic respiratory diseases.61
RSVRSV vaccination is recommended in patients with other chronic respiratory diseases (Recommendation B)CDC advises adults over 60—especially those with chronic lung disease or weakened immunity—to consider RSV vaccination.48 RSV vaccines should be part of adult immunization programs with age and risk prioritization.95 This ensures broader and more effective protection, especially in older adults.
In children under 2 years with chronic respiratory disease, monoclonal antibodies (nirsevimab) have proven safe and effective, even in a second season.96,97 Several countries, including Spain, recommend immunization before the epidemic season.61
B. pertussisPertussis vaccination is recommended in adults with other chronic respiratory diseases (Recommendation B)Pertussis can worsen respiratory conditions like bronchiectasis. The Tdap vaccination is advised for patients with chronic respiratory diseases,95 and has proven effective in reducing infections in the general population.98
Herpes zosterHerpes zoster vaccination is recommended in adults with other chronic respiratory diseases (Recommendation C)Chronic respiratory diseases increase herpes zoster risk and its complications at any age. This risk is higher in patients using inhaled corticosteroids.50,51 The CDC recommends herpes zoster vaccination in people aged 50 or older.64
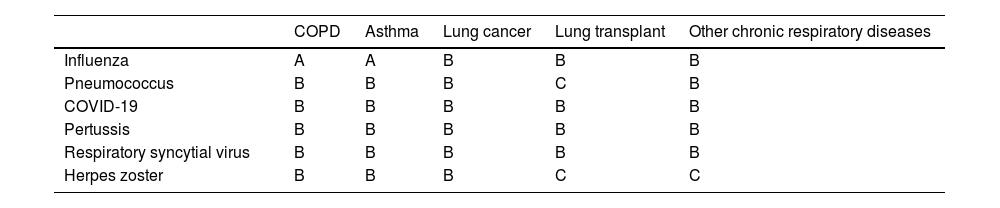
ConclusionVaccines play a key role in public health by preventing serious infections, especially in vulnerable populations such as individuals with chronic respiratory diseases (Table 1). Widespread vaccination reduces disease burden, improves quality of life, and lowers mortality. Ensuring access and promoting vaccination in these at-risk groups is a top priority for managing chronic respiratory disease, preventing complications, and improving health outcomes.
Summary of vaccination recommendations in different chronic respiratory diseases, with indication of the level of evidence.a
| COPD | Asthma | Lung cancer | Lung transplant | Other chronic respiratory diseases | |
|---|---|---|---|---|---|
| Influenza | A | A | B | B | B |
| Pneumococcus | B | B | B | C | B |
| COVID-19 | B | B | B | B | B |
| Pertussis | B | B | B | B | B |
| Respiratory syncytial virus | B | B | B | B | B |
| Herpes zoster | B | B | B | C | C |
Levels of evidence: evidence A, based on high-quality randomized clinical trials or robust meta-analyses; evidence B, based on smaller controlled studies or strong observational studies; evidence C, based on uncontrolled studies or case series; and evidence D, based primarily on expert opinions or clinical consensus.
COPD: chronic obstructive pulmonary disease.
This manuscript has not received any funding.
Conflicts of interest- -
JDM has received honoraria and funding from AstraZeneca, BIAL, Boehringer, Chiesi, FAES, Gebro, GSK, Janssen, Menarini, MSD, Novartis, Pfizer, Roche, Sanofi, Teva, and Zambon.
- -
RJC declares no conflicts of interest for this document.
- -
FSH declares no conflicts of interest for this document.
- -
RMV has received honoraria and funding from Pfizer, GSK, Sanofi, Novavax, and MSD.
- -
BSG declares no conflicts of interest for this document.
- -
SQF reports honoraria for lectures from GSK.
- -
RPR declares no conflicts of interest for this document.
- -
VMMC declares no conflicts of interest for this document.
- -
MEC has received honoraria and funding from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, FAES, Gebro, GSK, Menarini, MSD, Sanofi, Teva, and Zambon.
- -
LSF declares no conflicts of interest for this document.
- -
MMCM has received honoraria from Pfizer and funding for courses and congresses from Pfizer, Chiesi, Boehringer, and Teva.
- -
OM works as a consultant for Eli Lilly and Resmed.
- -
AGO has received honoraria and funding from AstraZeneca, BIAL, GSK, Janssen, Menarini, and MSD.
- -
JLGR reports honoraria for lectures from Gebro Pharma, GSK, AstraZeneca, Chiesi, Grifols, and Sanofi; consulting fees from GSK, AstraZeneca, Grifols, ALK, and Sanofi; and research grants from AstraZeneca.
- -
DRC has received honoraria and funding from AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, Gebro, Grifols, GSK, Insmed, Menarini, Praxis, TEVA, and Zambon.