The purpose of this document is to establish SEPAR's official position on the use of high-flow nasal cannula (HFNC) therapy in the home management of patients with chronic respiratory diseases. This position statement is deemed necessary considering current evidence regarding HFNC use in chronic respiratory conditions, with the objective of standardizing its application. This consensus was developed by a panel of experts comprising specialists with established expertise in chronic respiratory failure and high-flow nasal cannula therapy. The panel of experts stablished recommendations in COPD, bronchiectasis, interstitial lung diseases, palliative care, rehabilitation, and chronic treatment settings.
The purpose of this document is to establish SEPAR's official position on the use of high-flow nasal cannula (HFNC) therapy in the home management of patients with chronic respiratory diseases. This position statement is deemed necessary considering current evidence regarding HFNC use in chronic respiratory conditions, with the objective of standardizing its application.
While the role of HFNC in acute respiratory failure is well-established,1 the clinical evidence supporting its prolonged use at home remains limited. This position statement is founded on a comprehensive review of existing scientific literature concerning the efficacy and safety of HFNC therapy in the home environment, alongside a consensus among clinical experts. It also provides recommendations to assist healthcare professionals in utilizing this therapy for patients with chronic respiratory diseases such as COPD, bronchiectasis, interstitial lung disease (ILD), and in palliative care contexts.
Chronic respiratory diseases, including Chronic Obstructive Pulmonary Disease (COPD), are among the leading causes of morbidity and mortality worldwide.2,3 Together with bronchiectasis4,5 and interstitial lung disease,6 they constitute a significant burden in terms of morbidity and mortality among respiratory patients. In advanced stages, with chronic respiratory failure, management has traditionally relied on conventional oxygen therapy (COT) and more recently on home non-invasive ventilation (NIV). However, both modalities have notable limitations regarding outcomes and patient adherence,7 prompting the exploration of more effective alternatives.
In this context, HFNC has emerged as a promising intervention for the home management of patients with chronic respiratory diseases, due to its capacity to improve ventilation, enhance mucociliary clearance, and reduce the work of breathing.8,9 Additionally, there is accumulating evidence supporting its role in reducing exacerbations10,11 and hospitalizations,12 as well as enhancing patient comfort and quality of life.10 Despite these promising findings, uncertainties persist regarding the optimal integration of HFNC into the management of chronic respiratory diseases. Key considerations include patient selection, appropriate indications, combinations with other therapies, and therapeutic escalation, as HFNC is not a universal treatment for all patients with chronic respiratory failure.13 Results from ongoing controlled trials and real-world studies14 are crucial to further elucidate the effectiveness of HFNC in this patient population.
MethodologyThis consensus was developed by a panel of experts comprising specialists with established expertise in chronic respiratory failure and high-flow nasal cannula therapy. All the methodology of work is explained in the online supplement. Final recommendations are in Tables 1 and 2.
HFNC is a non-invasive respiratory support method that delivers heated and humidified oxygen-enriched air at flow rates exceeding 15L/min.1
Physiological EffectsThe physiological effects of HFNC are well-documented, and in the context of home and chronic care, they include:
- •
Improved mucociliary clearance15: It is well known that the respiratory epithelium is sensitive to pressure and humidity to maximize its function. Low humidity, as generated by conventional oxygen flows, can alter the viscosity of respiratory secretions and impair mucociliary clearance and ciliary function. Additionally, low inhaled air temperatures can induce bronchoconstriction in patients with COPD, bronchiectasis, or asthma.
- •
Dead space washout16: It may be less relevant in the home care setting compared to acute care because patients in a home setting typically have a lower respiratory rate, which improves exhalation time and reduces dead space re-inhalation. This effect is closely related to CO2 clearance, although the lower flow rates used in home care may limit its benefit.
- •
Stable FiO2: In situations requiring high flows, HFNC can provide stable FiO2. However, this is less relevant in home care since the high FiO2 levels needed in acute care are generally unnecessary.
- •
Counterbalancing intrinsic PEEP: In acute care, high flow rates are required to achieve oropharyngeal positive end-expiratory pressure (PEEP) to counterbalance intrinsic PEEP. In home care, lower flow rates may suffice as air trapping is typically less severe than during an exacerbation.17 Care should be taken to avoid excessive flow rates that might lead to undesirably high alveolar pressures in stable COPD patients.18,19 Finally, the PEEP-related benefits of HFNC may be diminished when used with a tracheostomy, as the PEEP effect is largely generated at the nasopharyngeal level due to the interaction of patient expiratory flow and continuous high flow from the device.20
- •
Reducing work of breathing: HFNC reduces the work of breathing by decreasing respiratory rate and accessory muscle use or by directly lowering the work of breathing as measured by transpulmonary pressure. This effect is evident in acute care settings.9,21–23 Preliminary research suggests a reduction in neural respiratory drive in home care settings with promising results.21
- •
Improvement in pulmonary mechanics21: HFNC can enhance pulmonary mechanics, including improvements in end-expiratory lung volume, compliance, and air distribution homogeneity, which are well-established in acute care. In home care, HFNC has shown improvements in pulmonary function, such as increased FEV1 and FVC in patients with bronchiectasis.24 In stable COPD patients and healthy volunteers, HFNC reduces respiratory rate and increases tidal volume, promoting a deeper and slower breathing pattern compared to COT.22 This can be shown by reducing ventilation inhomogeneity, increasing end-expiratory lung volumes, improving lung compliance and improving oxygenation and hypercapnia.23
The primary goals of HFNC in the home setting are to reduce the work of breathing and improve patient comfort and quality of life. For patients with chronic respiratory diseases, HFNC aims to manage symptoms, prevent exacerbations, and enhance overall respiratory function. By maintaining optimal humidity levels, HFNC supports mucociliary function and reduces the risk of respiratory infections.
Physiological RecommendationsBased on current evidence, HFNC is a promising option for home use in patients with chronic respiratory diseases. Key physiological effects include:
- •
Improving lung mechanics, leading to a deeper and slower breathing pattern.
- •
Decreasing neural respiratory drive, allowing muscular rest.
- •
Enhancing gas exchange and reducing hypercapnia.
- •
Improving mucus clearance.
- •
Increasing tolerance compared to conventional oxygen therapy.
As previously discussed, from a physiological perspective, HFNC can be beneficial for patients with stable COPD when used long-term at home.15–17,21,25 Based on the physiological effects, studies evaluating the impact of HFNC on stable COPD have focused on three primary outcomes: the number of exacerbations and hospitalizations, baseline PaCO2 levels, and quality of life, physical activity, dyspnea, and lung function.17
Clinical Evidence for Chronic Use of HFNC in Stable COPD- •
Effects of HFNC on reducing exacerbations: Three randomized controlled trials have evaluated the effect of HFNC on COPD exacerbations compared to conventional oxygen therapy. A study published in 201026 randomized 108 patients with COPD or bronchiectasis who had more than two exacerbations in the previous year to receive either high-flow therapy or conventional oxygen for one year. The time to the first exacerbation was significantly longer in the group treated with high-flow therapy. The number of exacerbations over one year was also lower in the HFNC group, although this did not reach statistical significance. Despite the results, the average usage of HFNC in this study did not exceed 2h per day. Nagata et al.27 also evaluated the time to the first exacerbation in 104 patients with COPD and chronic hypercapnia, compared conventional oxygen therapy with at least 4 daily hours of HFNC at approximately 30L/min. Patients treated with conventional oxygen alone experienced more than twice the number of exacerbations compared to those treated with HFNC. Two additional studies showed similar results. A 2018 study from Denmark,28 which included 200 patients with COPD and chronic respiratory failure, treated with nocturnal HFNC and daytime conventional oxygen or only conventional oxygen for one year, also demonstrated a reduction in the number of exacerbations in the HFNC group (3.12 exacerbations per year in the high-flow+oxygen group vs. 4.95 in the oxygen-alone group). A post hoc analysis of this study, published in 2019,29 indicated that the reduction in exacerbations was particularly significant in patients with more than two exacerbations in the previous year, suggesting that those with frequent exacerbations may derive the most benefit from this therapy.
- •
Effects of HFNC on reducing hospitalizations: Evidence regarding the impact of HFNC on hospitalizations is more limited. The Danish study by Storgaard et al.28 evaluated hospital admissions as a secondary endpoint, showing a reduction in hospital admissions in the HFNC group, although this did not reach statistical significance. A post hoc analysis of the study, published in 2020, revealed that the group treated with HFNC experienced a statistically significant reduction in hospital admissions in the year following treatment initiation.30 Recently a prospective multicenter study including 27 COPD exacerbators GOLD III and IV patients treated a year with home HFNC have showed decreased in exacerbations rate, hospital admissions, and in-hospital days.31
- •
Effects of HFNC on baseline PaCO2: Several studies have examined the potential of HFNC to reduce baseline PaCO2 in hypercapnic stable COPD patients. Studies comparing baseline PaCO2 in hypercapnic COPD patients treated with HFNC versus COT generally show improvements in PaCO2 associated with HFNC. One study randomized 74 patients with chronic hypercapnic COPD to conventional oxygen therapy or HFNC and assessed PaCO2 at 6 months and after a year.30 Baseline PaCO2 was lower in the HFNC group at both 6 months and one year. In each assessment, patients were connected to HFNC for 30min, and PaCO2 was measured before and after the session, showing decreases in PaCO2 in that time. The Nagata et al.32 study, which had a secondary objective of analyzing both baseline and nocturnal PaCO2, reported a reduction of 4.1mmHg in baseline PaCO2 and 5.1mmHg in nocturnal PaCO2. In 2019, Bräunlich et al.33 published a randomized crossover study involving 94 patients with COPD and a mean baseline PaCO2 of 56mmHg. Patients were consecutively treated with HFNC combined with daytime conventional oxygen, and nocturnal NIV combined with daytime COT. PaCO2 levels decreased by 7.1% with NIV and by 4.7% with HFNC, both statistically significant reductions. The authors concluded that HFNC could be a viable alternative for patients who do not tolerate NIV.
- •
Effects of HFNC on quality of life, dyspnea, exercise capacity, and lung function: Most studies evaluating HFNC in stable COPD include secondary objectives analyzing its impact on quality of life, dyspnea, exercise capacity, and lung function.28,29,32,33 Overall, improvements in quality of life are consistently observed. These outcomes will be further reviewed in the section on chronic use of HFNC in rehabilitation.
- •
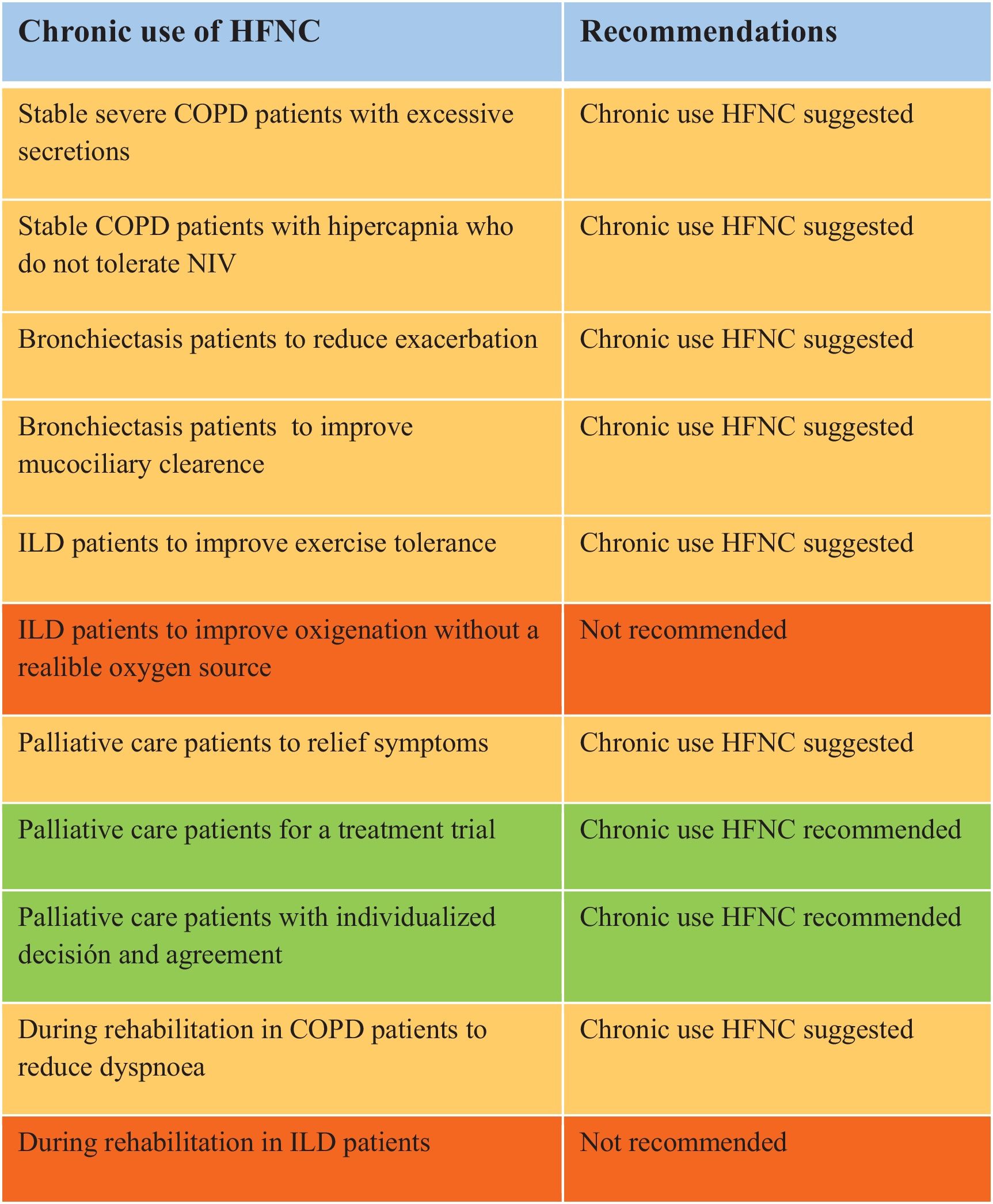
The group suggests the use of HFNC in stable severe COPD patients with excessive secretions (chronic bronchitis) to reduce exacerbations, improve control of chronic bronchial infection, and enhance quality of life. The evidence regarding its impact on reducing hospital admissions is less conclusive.
- •
The group suggests the use of HFNC in stable COPD patients with hypercapnia who do not tolerate NIV, with the objective of improving baseline PaCO2.
In bronchiectasis, chronic inflammation and persistent mucus retention drive recurrent exacerbations and contribute to airway damage.34–36 The physiological effects of HFNC can be beneficial for patients with bronchiectasis when used long-term at home.
HFNC is useful for interrupting the cycle of recurrent infections and reducing airway obstruction caused by mucus plugs.11,37 Furthermore, HFNC reduces the work of breathing and improves gas exchange, which is particularly advantageous for patients with severe disease.26,37 Consequently, the introduction of HFNC has shown promise, especially in reducing exacerbations and improving quality of life.
Clinical Evidence for Chronic Use of HFNC in BronchiectasisSeveral studies have demonstrated the benefits of long-term HFNC therapy in bronchiectasis. Simioli et al. in 202337 showed that long-term HFNC significantly reduced the frequency of acute exacerbations and hospitalizations in patients with both primary and secondary bronchiectasis. In a cohort of 78 patients, the mean number of exacerbations decreased from 2.81 to 0.45 over two years, with significant improvements in dyspnea scores, although no significant changes were observed in lung function parameters such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Similarly, a study by Crimi et al.24 demonstrated that HFNC use in bronchiectasis patients resulted in a significant reduction in exacerbation rates and hospitalizations, along with improvements in lung function markers such as FEV1 and FVC. A post hoc analysis by Good et al.38 further emphasized the efficacy of HFNC in reducing exacerbation rates in patients with stable bronchiectasis, showing a 31.3% reduction in exacerbations compared to usual care and significant improvements in quality of life as measured by the St. George's Respiratory Questionnaire. Additionally, Rea et al. in 201026 confirmed that HFNC therapy prolonged the time to first exacerbation and reduced the number of exacerbation days in patients with both chronic COPD and bronchiectasis, with more patients remaining exacerbation-free, indicating its utility in managing these chronic respiratory conditions. Recently, a total of 86 non-cystic fibrosis bronchiectasis patients with at least one severe exacerbation in the previous year were enrolled in one-year prospective study. The results showed that long-term HFNT reduces the annual exacerbation rate.39
Adherence to long-term HFNC is generally high, with most studies reporting usage rates between 5 and 8h per day. HFNC is well tolerated, with minimal side effects reported. Safety data from these studies suggest that the therapy is associated with few adverse events, making it a viable option for home use.11,37,40
Recommendations for Chronic Use of HFNC in Bronchiectasis- •
The group suggests the use of HFNC for patients with bronchiectasis to reduce the frequency of exacerbations, improve quality of life, and potentially enhance lung function.
- •
The group suggests the use of HFNC to improve mucociliary clearance and reduce the work of breathing. It makes HFNC particularly valuable in managing the chronic symptoms of bronchiectasis.
Interstitial lung diseases (ILD) are characterized by a variable and often progressive course, with symptoms such as exertional dyspnea.41 The disease course may be complicated by acute respiratory failure and, in advanced stages, by chronic respiratory failure. In the acute setting, HFNC has been shown to be superior to COT and not inferior NIV in treating severe hypoxemic acute respiratory failure, offering better comfort and patient perception.42,43 However, limited data are available on the use of HFNC in chronic and home settings, particularly in ILD.11,44
Clinical Evidence for Chronic Use of HFNC in Interstitial Lung DiseasesWeinreich et al. conducted a pilot crossover study of domiciliary HFNC in 9 ILD patients already receiving either ambulatory oxygen therapy or long-term oxygen therapy over a 6-week period.45 Patients were instructed to use the HFNC for 8h a day, preferably during nighttime, at a flow rate of 30L/min, with oxygen flow at their previously prescribed level (FiO2 on HFNC at 0.26). Patients used HFNC for an average of 6.5h per day, which was associated with improvements in the 6-minute walk test (6MWT) distance (441 vs. 393m) and breathlessness (mMRC score). No significant effects were observed on lung function, blood gas analyses, or quality of life.
HFNC has also been shown to improve exercise tolerance better than COT in ILD patients with exertional desaturation, as evidenced by increased endurance time during a cardiopulmonary exercise test.46–49 An inverse correlation was found between mean SpO2 values during the baseline room air 6MWT and improvements in endurance time with HFNC, suggesting that HFNC might be particularly beneficial for patients with greater exertional hypoxemia.46 These findings support the potential use of HFNC during pulmonary rehabilitation, although further studies are needed to explore its application in home settings.50
HFNC has also been used in end-of-life and palliative care settings for ILD patients, where management often focuses on symptom relief and improving quality of life, facilitating discharge from acute care facilities.51
However, a recent study published in late 2024 showed that adherence to home HFNC in patients with ILD was low, possibly due to the limited impact of this treatment on symptom relief.52 Although the expected benefits of HFNC in ILD patients include improved exercise capacity and reduced breathlessness, given the importance of correcting desaturation, a significant challenge remains ensuring a home oxygen source, such as concentrators or liquid oxygen, capable of delivering the high FiO2 required during exercise and in end-stage lung disease.
Recommendations for Chronic Use of HFNC in Interstitial Lung Diseases- •
The group suggests the use of HFNC for ILD patients to improve exercise tolerance, though the current evidence is of low grade.
- •
The group doesn’t recommend using HFNC without a reliable oxygen source, capable of delivering an adequate flow rate for patients with high FiO2 requirements.
The effects of HFNC at home may meet the criteria to be considered a suitable end-of-life treatment53–56 due to its physiological benefits53,54,56–58 and user-friendly characteristics.12,51,59,60 However, most evidence supporting its use comes from outside the palliative care context.
Clinical Evidence for Chronic Use of HFNC in Palliative CareStudies focusing on the use of HFNC at home during the end-of-life stage are limited.54,61 Dolidon et al.51 conducted a retrospective study involving 71 patients with terminal respiratory failure from various aetiologies, demonstrating that HFNC therapy enabled patients to be discharged and remain at home with acceptable survival rates and reasonable costs. In elderly populations60 or those with a Do Not Intubate order,62–65 HFNC has shown positive effects, though key aspects remain unclear. Most studies compare HFNC with conventional treatments:
- •
HFNC vs. COT: A hospital-based study66 demonstrated the superiority of HFNC in reducing dyspnea within the first hour in patients with a Do Not Intubate order and hypoxemic respiratory failure.
- •
HFNC vs. NIV: Hospital studies suggest similar benefits with better tolerance for HFNC.67 Peters et al.,63 in a retrospective observational study, supported the use of HFNC over NIV for adequate oxygenation in patients with terminal respiratory disease and hypoxemic respiratory failure under a Do Not Intubate order. These findings may be extrapolated to home settings. Other studies found HFNC at the same level of NIV benefits, with better tolerance.51,68,69 However, only the French study47 offers a domiciliary perspective. In cases of ILD and oncological diseases with respiratory involvement, HFNC significantly improved oxygenation and dyspnea and was well tolerated.63,65–67,70,71
The goals of palliative HFNC include optimizing opioid use for symptom control, maintaining oxygenation levels at home, reducing the need for prolonged hospitalization, and enabling oral communication and feeding. HFNC provides a less traumatic interface, easing the burden on caregivers.72 Huang et al.64 recommend a flow rate of 20L/min over seven to eight hours, preferably at night.
Recommendations for Chronic Use of HFNC in Palliative Care- •
The group suggests the use of HFNC to a symptomatic treatment strategy for end-of-life patients with respiratory disease, with the potential for symptom relief, improved quality of life, and avoidance of unwanted hospitalization.
- •
The group recommends using HFNC for a treatment trial to evaluate symptom relief and ensure the absence of adverse effects or harm.
- •
The group recommends using HFNC for palliative care with a individualized decision and with the agreement of the patient and their family, with clear communication of the treatment goals.
HFNC offers theoretical potential benefits in exercise training and pulmonary rehabilitation for patients with chronic respiratory diseases, primarily COPD and ILD. These benefits include reducing muscle load and maintaining a constant FiO2 despite high oxygen demands during exercise.
Clinical Evidence for Use of HFNC During Rehabilitation in COPD PatientsIn COPD patients, HFNC can alleviate hyperinflation, reduce the work of breathing, and decrease dyspnea and fatigue during exercise training. HFNC achieves these effects by reducing respiratory muscle load, lowering respiratory rate, and prolonging expiratory time.73 Chao et al. conducted a single crossover trial showing that HFNC improved self-paced exercise performance, with a statistically significant increase of 27.3m in the 6MWT compared to COT. However, this improvement was slightly below the threshold for clinical significance, with no differences observed in dyspnea, blood pressure, respiratory rate, or heart rate between the two groups.74
In a small study, Cirio et al. found that using HFNC during constant-load exercise testing in COPD patients improved exercise time and oxygen saturation, while reducing perceptions of dyspnea and muscle fatigue compared to the Venturi mask, suggesting HFNC may enhance training endurance even without supplemental oxygen.75
Vitacca et al. also observed a significant increase in 6MWT, although endurance time improvements were not statistically significant during pulmonary rehabilitation programs.76 A recent meta-analysis found that HFNC had little to no effect on quality of life, exercise capacity, or breathlessness during pulmonary rehabilitation in COPD patients, though improvements were noted in non-domiciliary oxygen patients.77
Clinical Evidence for Use of HFNC During Rehabilitation in ILD PatientsIn ILD patients, HFNC has shown potential to improve endurance time,46 though with no effects on 6MWT and limited effects on dyspnea. Harada et al., in a randomized controlled trial comparing HFNC and the Venturi mask, found that the HFNC group had increased endurance time, higher peripheral oxygen saturation, and reduced leg fatigue compared to the Venturi mask group. However, dyspnea, maximum heart rate, and comfort at 80% peak work rate were unaffected by HFNC.48 Badenes et al., in a single exercise test, also reported an increase in endurance time.46 Yanagita et al. recently suggested that HFNC without supplemental oxygen can increase SpO2 levels during exercise in mild ILD patients.49 In patients with lung cancer, a single study reported that HFNC significantly reduced dyspnea and increased endurance time when used at 100% FiO2.69
Recommendations for Use of HFNC During Rehabilitation- •
The group suggests the use of HFNC to improve performance in the 6MWT and reduce dyspnea in some COPD patients during rehabilitation, although its effect on endurance time is unclear, with minimal impact on other physiological parameters such as heart rate, respiratory rate, and blood pressure.
- •
The group doesn’t recommends using HFNC during rehabilitation in ILD patients because the evidence is limited and inconsistent, suggesting that HFNC may increase endurance time, but its effects on dyspnea remain minimal across reviewed studies.
Home use of HFNC may require a different approach than hospital use, as the goals can vary. Given its application across diverse conditions with varying pathophysiology, a personalized approach should be employed to titrate parameters effectively.
Setting the Flow in HFNCIn hospital settings, flow is titrated to match or exceed the patient's peak inspiratory flow. In chronic home-based care, several factors may influence the required flow.
- •
Most studies in patients with stable COPD have shown that even a minimum flow of 20L/min can generate physiological benefits, such as a reduction in neuro-respiratory drive.21
- •
Higher flows (>50L/min) may exacerbate air trapping in COPD, potentially leading to high near-alveolar pressures.18,19
- •
In stable settings, higher flows may be less well tolerated. Since the washout of dead space primarily occurs during exhalation, and stable patients typically have a lower respiratory rate, the benefits can be attained with lower flows than in acute care.
- •
Most studies in COPD have utilized flow rates between 20 and 30L/min.25–27
- •
In bronchiectasis, flows are typically set between 20 and 40L/min based on patient tolerance, with a mean tolerated flow of 33L/min.24
- •
In acute care, high flows are necessary to maintain a constant inspired FiO2. However, in home care, patients are more stable, with lower peak inspiratory flows and generally lower FiO2 requirements.78
- •
The goals for flow setting should include improvements in arterial blood gases and relief of dyspnea while ensuring patient tolerance.
- •
In most cases, flows between 20 and 30L/min are sufficient and well tolerated.
The standard goal is to maintain a temperature of 37°C, though this may not be well tolerated in hotter climates. Many trials set temperature targets between 34 and 37°C, aiming for optimal humidification at 37°C. A practical approach is to start at 31°C and gradually increase to 37°C as tolerated.
Setting FiO2 in HFNCFor home use, the management of severe hypoxemia is less of a focus compared to acute care, with supplemental oxygen requirements typically not exceeding 10–15L/min. Conventional oxygen sources (static concentrators) are usually limited to supplying 8–9L/min, while liquid oxygen can supply up to 15L/min. Thus, delivered FiO2 is often below 50%. Most home care devices are not approved for more than 15L/min of supplemental oxygen flow.
In COPD studies, supplemental oxygen flow rarely exceeded 2L/min.8,25–27 In bronchiectasis, many patients required no supplemental oxygen, with a mean FiO2 of 21%.24 The recommendation is to adjust supplemental oxygen to maintain SpO2 within the desired range (usually 90–92%).
Setting Patient Interface in HFNCThe recommended approach is to use nasal cannulas that occlude approximately 50% of the nostrils. There is currently no real-life data to support the preference of asymmetrical nasal cannulas over conventional ones in home care settings.
Setting Compliance to HFNC TreatmentStudies suggest that a mean usage of 6–8h per day is sufficient to achieve benefits, with some trials reporting usage exceeding 7h per day.26 It is recommended to use HFNC primarily during nocturnal hours and as long as tolerated unless combined with NIV.25
Recommendations for Setting and Titrating Parameters in Chronic HFNC- •
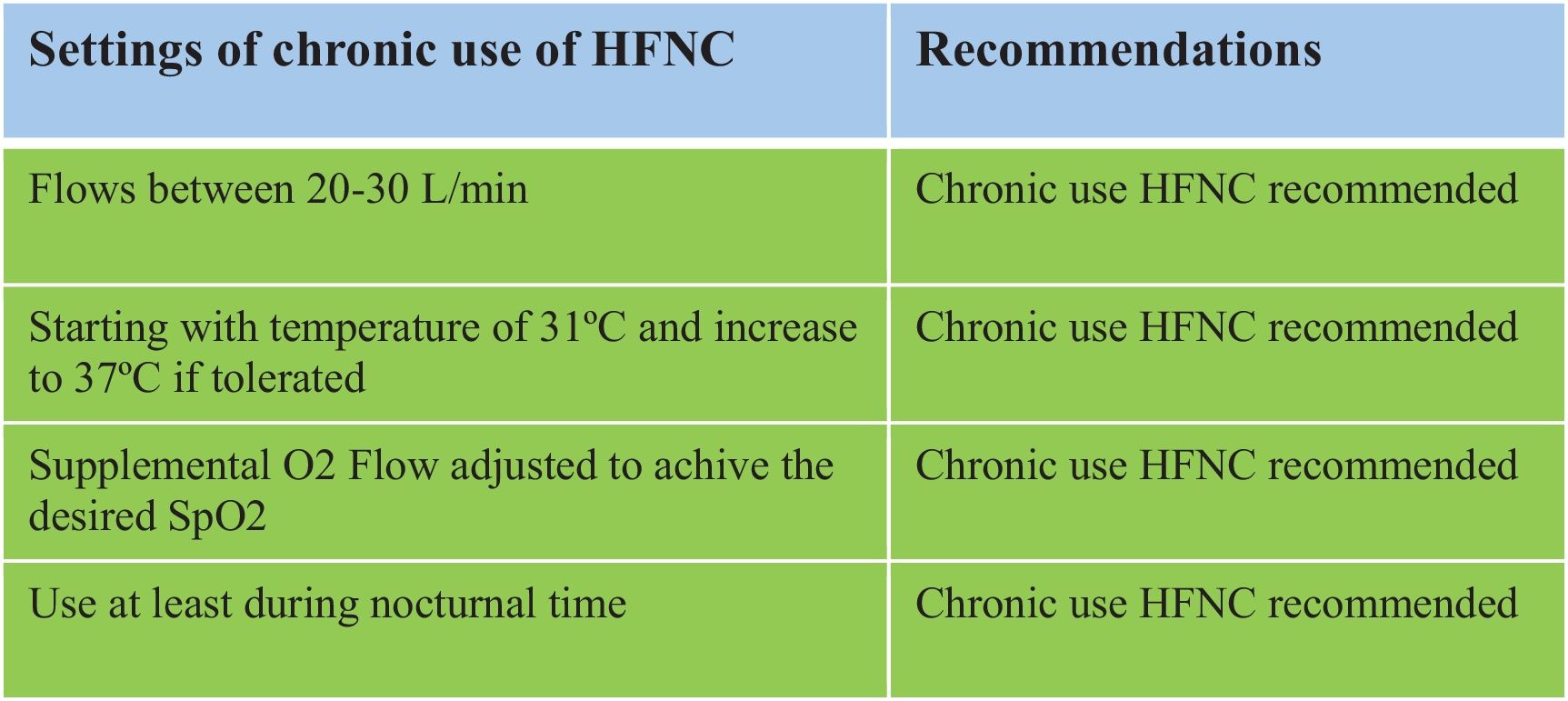
The group recommends using HFNC with flows between 20 and 30L/min for effective and well-tolerated treatment.
- •
The group recommends using HFNC starting with a temperature of 31°C and progressively increase to 37°C as tolerated.
- •
The group recommends using HFNC with a supplemental O2 flow adjusted to achieve the desired SpO2 range (usually 90–92%).
- •
The group recommends using HFNC at least during nocturnal hours.
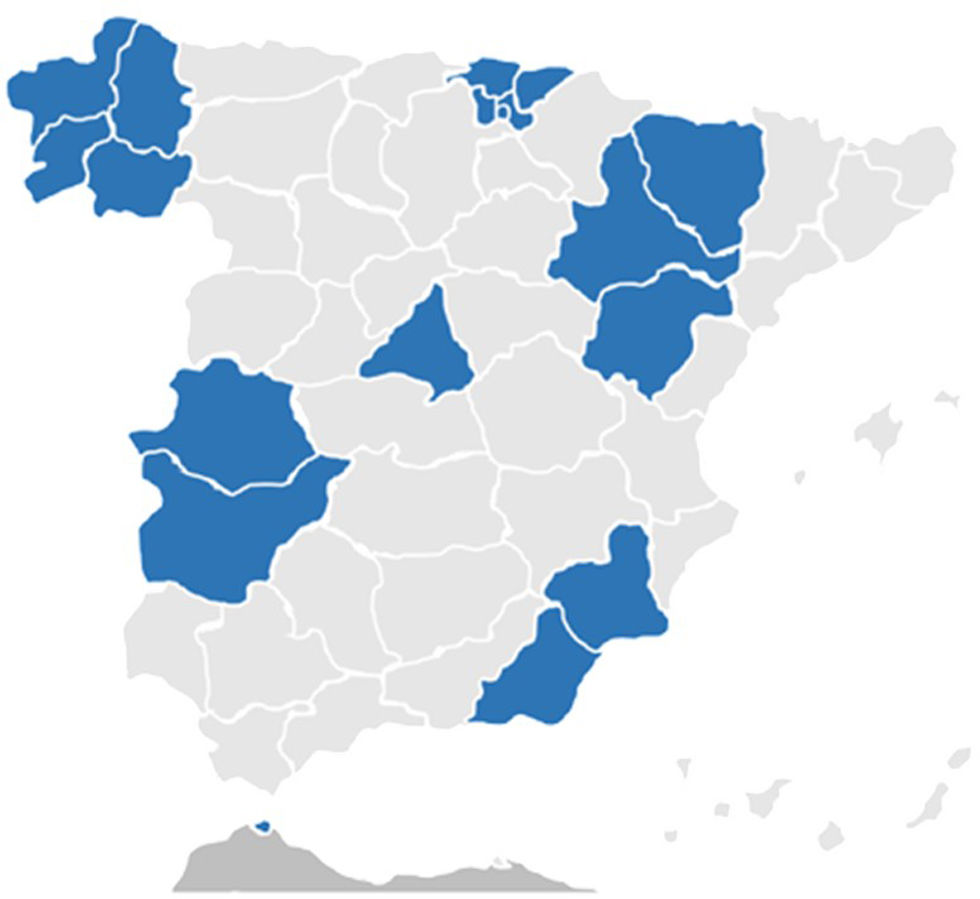
HFNC can be prescribed in various regions of Spain, although its implementation still lacks uniformity across the country.79 To facilitate its widespread use, HFNC must be included in the technical specifications of tenders for home respiratory therapies or offered as an improvement by the winning home care provider. This allows its inclusion even if it is not explicitly mentioned in the initial tender.
The fact that public tenders for home respiratory therapies, where HFNC is included, are drafted by regional health administrations80 in collaboration with the scientific community—establishing the financing model, conditions, and quality criteria for the service—leads to significant heterogeneity in these aspects, resulting in disparities in services provided to patients across different regions.81
From 2018 to 2021, approximately half of the published tenders included the possibility of prescribing HFNC (e.g., Aragón through a special agreement, Ceuta, Galicia, Madrid, and Murcia),82–85 while others excluded it (e.g., Catalonia, Cantabria, Jaén, Córdoba, Melilla, and the Balearic Islands).86–88 Starting in 2022, there has been a noticeable shift, with nearly all tenders (e.g., Aragón, Almería, Extremadura, the Basque Country, and Cantabria)89–93 incorporating HFNC. However, Málaga (published in 2022)94 and Castilla y León95 (pending completion) have not yet included it. Full implementation will depend on the progressive update of various tenders (Fig. 1).
Recommendations for HFNC Therapy Prescription in Spain- •
It is recommended to review the technical specifications or the winning tender for the relevant region to gain a detailed understanding of the services provided and assess whether HFNC is included.
The lack of meta-analyses and the heterogeneity of available studies, comprising a mix of randomized controlled trials and lower-quality observational studies, constrain the strength of evidence supporting the use of home high-flow nasal cannula (HFNC). Notably, there is a current shortage of multicenter studies in this field. Furthermore, no studies have conducted a cost-benefit analysis assessing the economic impact of home HFNC use. As this represents an emerging technology, it is crucial to demonstrate a clear balance between cost and clinical benefit. As we know, there is only a guideline published about this subject, the chronic HFNC Danish guideline.96 It is reviewed too in a chapter of long-term oxygen therapy Australia and New Zealand guideline.97 Therefore, the aim of this document is to establish SEPAR's initial position on the use of these therapies, acknowledging that the development of a clinical practice guideline will require more robust future evidence.
ConclusionIn conclusion, the chronic use of HFNC at home represents a novel treatment that could aid in managing patients with chronic respiratory diseases, particularly those with frequent exacerbations or poor secretion management. It may also be beneficial in the palliative care of respiratory patients. Additionally, the chronic use of HFNC could positively impact the exercise capacity of these patients. However, the current scientific evidence on this subject remains limited, and further studies are required to better elucidate the utility of this therapy. The results of two ongoing studies involving patients with COPD are expected soon.98,99 Regarding the prescription of HFNC in Spain, significant heterogeneity has been observed across regions, underscoring a clear area for improvement.